Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro - PubMed (original) (raw)
. 2021 Sep 23;13(10):1914.
doi: 10.3390/v13101914.
Mira Alt 1, Leonie Schipper 1, Lukas van de Sand 1, Vu Thuy Khanh Le-Trilling 2, Lydia Rink 2, Natalie Heinen 3, Rabea Julia Madel 1, Mona Otte 1, Korbinian Wuensch 1, Christiane Silke Heilingloh 1, Thorsten Mueller 4 5, Ulf Dittmer 2, Carina Elsner 2, Stephanie Pfaender 3, Mirko Trilling 2, Oliver Witzke 1, Adalbert Krawczyk 1 2
Affiliations
- PMID: 34696344
- PMCID: PMC8537626
- DOI: 10.3390/v13101914
Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro
Maren Bormann et al. Viruses. 2021.
Abstract
Severe Acute Respiratory Syndrome Coronavirus Type 2 (SARS-CoV-2) is the causative agent of the coronavirus disease 2019 (COVID-19). The availability of effective and well-tolerated antiviral drugs for the treatment of COVID-19 patients is still very limited. Traditional herbal medicines elicit antiviral activity against various viruses and might therefore represent a promising option for the complementary treatment of COVID-19 patients. The application of turmeric root in herbal medicine has a very long history. Its bioactive ingredient curcumin shows a broad-spectrum antimicrobial activity. In the present study, we investigated the antiviral activity of aqueous turmeric root extract, the dissolved content of a curcumin-containing nutritional supplement capsule, and pure curcumin against SARS-CoV-2. Turmeric root extract, dissolved turmeric capsule content, and pure curcumin effectively neutralized SARS-CoV-2 at subtoxic concentrations in Vero E6 and human Calu-3 cells. Furthermore, curcumin treatment significantly reduced SARS-CoV-2 RNA levels in cell culture supernatants. Our data uncover curcumin as a promising compound for complementary COVID-19 treatment. Curcumin concentrations contained in turmeric root or capsules used as nutritional supplements completely neutralized SARS-CoV-2 in vitro. Our data argue in favor of appropriate and carefully monitored clinical studies that vigorously test the effectiveness of complementary treatment of COVID-19 patients with curcumin-containing products.
Keywords: COVID-19; Curcuma longa; SARS-CoV-2; antiviral; curcumin; herbal medicine; turmeric root.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Figures
Figure 1
Neutralization of SARS-CoV-2 by aqueous turmeric root extract, curcumin-containing nutritional supplement capsules, and curcumin. Decreasing concentrations of aqueous turmeric root extract (1:8–1:1024 dilution), nutritional supplement capsules (468.8–3.7 µg/mL), and curcumin (125–1 µg/mL) were pre-incubated with 100 TCID50 of SARS-CoV-2 for one hour and subsequently added to confluent Vero E6 cells. After 48 h, cells were stained with crystal violet and analyzed for cytopathic effects using transmitted light microscopy. The experiment was performed three times independently. Representative images are displayed. NC = negative control (medium); PC = positive control (100 TCID50 SARS-CoV-2); scale bar = 200 µm.
Figure 2
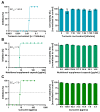
Dose-dependent antiviral activity of aqueous turmeric root extract, curcumin-containing nutritional supplement capsules, and curcumin against SARS-CoV-2. Decreasing concentrations of aqueous turmeric root extract (1:8–1:1024 dilution) (A), nutritional supplement capsules (468.8–3.7 µg/mL) (B), and curcumin (125–1 µg/mL) (C) were pre-incubated with 100 TCID50 of SARS-CoV-2 for one hour. Subsequently, each dilution of virus–herb suspensions was incubated on confluent Vero E6 cells grown on a 96-well plate. After 48 h, cells were stained with crystal violet and analyzed regarding cytopathic effects. The half-maximal effective concentration (EC50) was calculated by nonlinear regression using GraphPad Prism. The cytotoxic effect of various concentrations of aqueous turmeric root extract, nutritional supplement capsules, and curcumin toward Vero E6 cells was determined by Orangu Cell Counting Solution (Cell guidance systems) after 48 h. Cell viability was normalized to untreated control cells. The experiment was performed three times independently. Error bars represent the standard error of the mean (SEM).
Figure 3
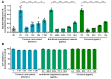
Neutralization of SARS-CoV-2 by aqueous turmeric root extract, curcumin-containing nutritional supplement capsules, and curcumin on a human cell line assessed by an in-cell ELISA (icELISA)-based neutralization test (icNT). (A) Decreasing concentrations of aqueous turmeric root extract (1:16–1:128 dilution), nutritional supplement capsule content (468.8–58.6 µg/mL), or curcumin (125–15.6 µg/mL) were pre-incubated with 5000 plaque-forming units (PFU) of SARS-CoV-2 for one hour. Subsequently, mixtures were added to human Calu-3 cells and incubated for 24 h. After incubation with a SARS-CoV-2 N-specific primary antibody and peroxidase-labelled secondary antibody, the enzyme reaction was visualized by adding tetramethylbenzidine. Absorbance was measured with a microplate multireader at OD450. Statistical analysis was performed with one-way analysis of variance (ANOVA) and Dunnett’s multiple comparison test using GraphPad Prism. ** p < 0.01; *** p < 0.001; and **** p < 0.0001; error bars represent the standard error of the mean (SEM). NC = negative control (medium); PC = positive control (5000 PFU SARS-CoV-2). (B) The cytotoxic effect of various concentrations of aqueous turmeric root extract, nutritional supplement capsule, and curcumin toward Calu-3 cells was determined by Orangu™ Cell Counting Solution (Cell guidance systems) after 24 h. The cell viability was normalized to untreated control cells. Error bars represent the SEM.
Figure 4
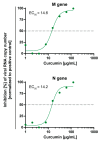
Dose-dependent activity of curcumin on SARS-CoV-2 RNA genome copy numbers. Decreasing concentrations of curcumin (125–1 µg/mL) were pre-incubated with 100 TCID50 of SARS-CoV-2 for one hour and subsequently added to confluent Vero E6 cells. After 48 h, cell culture supernatants were harvested, and the genomic SARS-CoV-2 RNA was quantified via RT-qPCR, using primer targeting the viral M or N gene. Cells infected with 100 TCID50 of SARS-CoV-2 served as positive control. The experiment was performed three times independently. The half-maximal effective concentration (EC50) was calculated by nonlinear regression using GraphPad Prism.
Similar articles
- Optimization of Anti-SARS-CoV-2 Treatments Based on Curcumin, Used Alone or Employed as a Photosensitizer.
Zupin L, Fontana F, Clemente L, Borelli V, Ricci G, Ruscio M, Crovella S. Zupin L, et al. Viruses. 2022 Sep 27;14(10):2132. doi: 10.3390/v14102132. Viruses. 2022. PMID: 36298687 Free PMC article. - Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms.
Marín-Palma D, Tabares-Guevara JH, Zapata-Cardona MI, Flórez-Álvarez L, Yepes LM, Rugeles MT, Zapata-Builes W, Hernandez JC, Taborda NA. Marín-Palma D, et al. Molecules. 2021 Nov 16;26(22):6900. doi: 10.3390/molecules26226900. Molecules. 2021. PMID: 34833991 Free PMC article. - Antiviral activity of oleandrin and a defined extract of Nerium oleander against SARS-CoV-2.
Plante KS, Dwivedi V, Plante JA, Fernandez D, Mirchandani D, Bopp N, Aguilar PV, Park JG, Tamayo PP, Delgado J, Shivanna V, Torrelles JB, Martinez-Sobrido L, Matos R, Weaver SC, Sastry KJ, Newman RA. Plante KS, et al. Biomed Pharmacother. 2021 Jun;138:111457. doi: 10.1016/j.biopha.2021.111457. Epub 2021 Mar 3. Biomed Pharmacother. 2021. PMID: 33721754 Free PMC article. - Effect of the Phytochemical Agents against the SARS-CoV and Some of them Selected for Application to COVID-19: A Mini-Review.
Idrees M, Khan S, Memon NH, Zhang Z. Idrees M, et al. Curr Pharm Biotechnol. 2021;22(4):444-450. doi: 10.2174/1389201021666200703201458. Curr Pharm Biotechnol. 2021. PMID: 32619167 Review. - Propolis, Bee Honey, and Their Components Protect against Coronavirus Disease 2019 (COVID-19): A Review of In Silico, In Vitro, and Clinical Studies.
Ali AM, Kunugi H. Ali AM, et al. Molecules. 2021 Feb 25;26(5):1232. doi: 10.3390/molecules26051232. Molecules. 2021. PMID: 33669054 Free PMC article. Review.
Cited by
- Introduction to Traditional Medicine and Their Role in Prevention and Treatment of Emerging and Re-Emerging Diseases.
Rizvi SAA, Einstein GP, Tulp OL, Sainvil F, Branly R. Rizvi SAA, et al. Biomolecules. 2022 Oct 9;12(10):1442. doi: 10.3390/biom12101442. Biomolecules. 2022. PMID: 36291651 Free PMC article. Review. - The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19-Results from a pragmatic randomized clinical trial.
Ujjan ID, Khan S, Nigar R, Ahmed H, Ahmad S, Khan A. Ujjan ID, et al. Front Nutr. 2023 Jan 18;9:1023997. doi: 10.3389/fnut.2022.1023997. eCollection 2022. Front Nutr. 2023. PMID: 36742008 Free PMC article. - Available and affordable complementary treatments for COVID-19: From hypothesis to pilot studies and the need for implementation.
Bousquet J, Haahtela T, Blain H, Czarlewski W, Zuberbier T, Bedbrook A, Cruz AA, Fonseca JA, Klimek L, Kuna P, Samolinski B, Valiulis A, Lemaire A, Anto JM. Bousquet J, et al. Clin Transl Allergy. 2022 Mar;12(3):e12127. doi: 10.1002/clt2.12127. Clin Transl Allergy. 2022. PMID: 35344297 Free PMC article. - Role of Curcuma longae Rhizoma in medical applications: research challenges and opportunities.
Zhang P, Liu H, Yu Y, Peng S, Zhu S. Zhang P, et al. Front Pharmacol. 2024 Aug 6;15:1430284. doi: 10.3389/fphar.2024.1430284. eCollection 2024. Front Pharmacol. 2024. PMID: 39170702 Free PMC article. Review. - Antiviral, anti-inflammatory and antioxidant effects of curcumin and curcuminoids in SH-SY5Y cells infected by SARS-CoV-2.
Nicoliche T, Bartolomeo CS, Lemes RMR, Pereira GC, Nunes TA, Oliveira RB, Nicastro ALM, Soares ÉN, da Cunha Lima BF, Rodrigues BM, Maricato JT, Okuda LH, de Sairre MI, Prado CM, Ureshino RP, Stilhano RS. Nicoliche T, et al. Sci Rep. 2024 May 10;14(1):10696. doi: 10.1038/s41598-024-61662-7. Sci Rep. 2024. PMID: 38730068 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous