The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases - PubMed (original) (raw)
Review
. 2022 Mar;26(3):671-683.
doi: 10.1007/s11605-021-05188-7. Epub 2021 Nov 3.
Ulrich Wirth 1, Dominik Koch 1, Malte Schirren 1, Moritz Drefs 1, Dionysios Koliogiannis 1, Hanno Nieß 1, Joachim Andrassy 1, Markus Guba 1, Alexandr V Bazhin 1, Jens Werner 1, Florian Kühn 2
Affiliations
- PMID: 34734369
- PMCID: PMC8926958
- DOI: 10.1007/s11605-021-05188-7
Review
The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases
Lingxuan An et al. J Gastrointest Surg. 2022 Mar.
Abstract
Background: Hepatosteatosis is the earliest stage in the pathogenesis of nonalcoholic fatty (NAFLD) and alcoholic liver disease (ALD). As NAFLD is affecting 10-24% of the general population and approximately 70% of obese patients, it carries a large economic burden and is becoming a major reason for liver transplantation worldwide. ALD is a major cause of morbidity and mortality causing 50% of liver cirrhosis and 10% of liver cancer related death. Increasing evidence has accumulated that gut-derived factors play a crucial role in the development and progression of chronic liver diseases.
Methods: A selective literature search was conducted in Medline and PubMed, using the terms "nonalcoholic fatty liver disease," "alcoholic liver disease," "lipopolysaccharide," "gut barrier," and "microbiome."
Results: Gut dysbiosis and gut barrier dysfunction both contribute to chronic liver disease by abnormal regulation of the gut-liver axis. Thereby, gut-derived lipopolysaccharides (LPS) are a key factor in inducing the inflammatory response of liver tissue. The review further underlines that endotoxemia is observed in both NAFLD and ALD patients. LPS plays an important role in conducting liver damage through the LPS-TLR4 signaling pathway. Treatments targeting the gut microbiome and the gut barrier such as fecal microbiota transplantation (FMT), probiotics, prebiotics, synbiotics, and intestinal alkaline phosphatase (IAP) represent potential treatment modalities for NAFLD and ALD.
Conclusions: The gut-liver axis plays an important role in the development of liver disease. Treatments targeting the gut microbiome and the gut barrier have shown beneficial effects in attenuating liver inflammation and need to be further investigated.
Keywords: ALD; Gut barrier; LPS; Microbiome; NAFLD.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Fig. 1
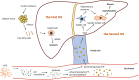
Histological zones of liver lobules (A) and the ductular reaction (B). A The liver can be divided functionally into three zones. Zone I is located around the portal triad, which is the most nutrient-oxygenated region. Zone III is located around the central vein, where oxygenation is poor. Zone II is located in between. B In chronic liver injury, hepatocyte regeneration is impaired and is replaced by the second pathway of HPCs activation. HPCs are bipotential cells and can differentiate into hepatocytes and cholangiocytes. The latter process causes the ductular reaction (DR). The HPCs can also interact with hepatic stellate cells, which are the primary source of the extracellular matrix (ECM) and the key players of the liver fibrogenic response
Fig. 2
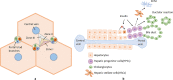
LPS acts as the second hit in the pathogenesis of NAFLD. Insulin resistance is the major factor for the progression of NAFLD, leading to an increase in free fatty acids (FFAs) in the circulating blood. Excessive uptake of FFAs by hepatocytes results in steatosis, making the liver more vulnerable to further insults, which is considered the “first hit.” A high-fat diet (HFD) could lead to gut dysbiosis, which further causes an increase in bacterial by-product production and increased gut permeability. Lipopolysaccharide (LPS) translocates the gut barrier, enters the liver through the portal vein, and activates Kupffer cells and hepatic stellate cells (HSCs) through the LPS-TLR4 pathway, resulting in an inflammatory response which leads to steatohepatitis and eventually fibrosis
Fig. 3
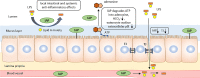
The role of IAP in preventing local inflammation, preventing LPS translocation, and regulation of enterocyte surface extracellular pH. IAP is highly expressed in the brush border membrane of duodenal epithelial cells and is secreted bilaterally into the gut lumen and the blood. IAP can detoxify LPS, resulting in amelioration of intestinal and systemic inflammation. ATP serves as a substrate for brush border IAP. In the gut lumen, the presence of ATP increases HCO3—secretion. IAP decreases luminal ATP concentration and diminish this pathway. IAP also plays a role in regulating tight junction protein levels, preserving gut integrity and preventing translocation of bacterial by-products
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials