Mitochondrial Electron Transport Chain Protein Abnormalities Detected in Plasma Extracellular Vesicles in Alzheimer's Disease - PubMed (original) (raw)
Mitochondrial Electron Transport Chain Protein Abnormalities Detected in Plasma Extracellular Vesicles in Alzheimer's Disease
Pamela J Yao et al. Biomedicines. 2021.
Abstract
Mitochondria provide energy to neurons through oxidative phosphorylation and eliminate Reactive Oxygen Species (ROS) through Superoxide Dismutase 1 (SOD1). Dysfunctional mitochondria, manifesting decreased activity of electron transport chain (ETC) complexes and high ROS levels, are involved in Alzheimer's disease (AD) pathogenesis. We hypothesized that neuronal mitochondrial dysfunction in AD is reflected in ETC and SOD1 levels and activity in plasma neuron-derived extracellular vesicles (NDEVs). We immunoprecipitated NDEVs targeting neuronal marker L1CAM from two cohorts: one including 22 individuals with early AD and 29 control subjects; and another including 14 individuals with early AD and 14 control subjects. In the first cohort, we measured levels of complexes I, III, IV, ATP synthase, and SOD1; in the second cohort, we measured levels and catalytic activity of complexes IV and ATP synthase. AD individuals had lower levels of complexes I (p < 0.0001), III (p < 0.0001), IV (p = 0.0061), and V (p < 0.0001), and SOD1 (p < 0.0001) compared to controls. AD individuals also had lower levels of catalytic activity of complex IV (p = 0.0214) and ATP synthase (p < 0.0001). NDEVs confirm quantitative and functional abnormalities in ECT complexes and SOD1 previously observed in AD models and during autopsy, opening the way for using them as biomarkers for mitochondrial dysfunction in AD.
Keywords: Alzheimer’s disease; NADH; SOD1; Superoxide Dismutase 1; electron transport chain; mitochondria; oxidative phosphorylation.
Conflict of interest statement
Goetzl has pending patent applications covering the basic exosome methods used in this publication. For the remaining authors, no competing financial interests exist.
Figures
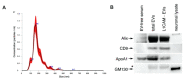
Figure 1
(A) NTA analysis of the size distribution of L1CAM-immunoprecipitated NDEVs. (B) Immunoblots of Alix, CD9, ApoA1, and GM130 in total EVs and NDEV samples. * Nonspecific band.
Figure 2
Levels of SOD1 and constituents of the mitochondrial oxidative phosphorylation system in plasma NDEVs of control and AD. Each data point represents the value for one study participant. The mean ± S.E.M. of control and AD groups, respectively, were 2252 ± 313 pg/mL and 769 ± 96 pg/mL, p < 0.001, for SOD1 (A); 1254 ± 135 pg/mL and 306 ± 40 pg/mL, p < 0.001, for subunit 1 of complex I (B); 972 ± 116 pg/mL and 558 ± 85 pg/mL, p < 0.01, for subunit 6 of complex I (C); 1692 ± 153 pg/mL and 399 ± 24 pg/mL, p < 0.001, for subunit 10 of complex III (D); 6209 ± 752 pg/mL and 3647 ± 513 pg/mL, p < 0.01, for subunit 1 of complex IV (E); 4863 ± 213 pg/mL and 2762 ± 221 pg/mL, p < 0.001, for ATP synthase (F). All values were normalized for content of the exosome marker CD81. Statistical significance of differences in values between control and AD groups were calculated by two sample t tests.
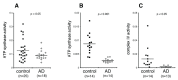
Figure 3
Activity of the mitochondrial oxidative phosphorylation proteins in plasma NDEVs of control and AD. Each data point represents the value for one study participant. (A), cohort 1; (B,C), cohort 2. The numbers of study participants are indicted on the graphs. The mean ± S.E.M. of control and AD groups, respectively, was 0.129 ± 0.015 and 0.088 ± 0.009, p < 0.05, for ATP synthase, cohort 1 (A); 0.009 ± 0.001 and 0.003 ± 0.0002, p < 0.001, for ATP synthase, cohort 2 (B); 0.013 ± 0.004 and 0.001 ± 0.0003, p < 0.05, for complex IV (C). Values in (A) were normalized for content of the EV marker CD81. Values in (B,C) were normalized for quantity of the respective protein that was determined within the same assay (see Methods). Statistical significance of differences in values between control and AD groups were calculated by two sample t tests.
Similar articles
- Mitochondrial dysfunction in a cell culture model of familial amyotrophic lateral sclerosis.
Menzies FM, Cookson MR, Taylor RW, Turnbull DM, Chrzanowska-Lightowlers ZM, Dong L, Figlewicz DA, Shaw PJ. Menzies FM, et al. Brain. 2002 Jul;125(Pt 7):1522-33. doi: 10.1093/brain/awf167. Brain. 2002. PMID: 12077002 - Mechanisms of Mitochondrial Dysfunction in Alzheimer's Disease.
Cadonic C, Sabbir MG, Albensi BC. Cadonic C, et al. Mol Neurobiol. 2016 Nov;53(9):6078-6090. doi: 10.1007/s12035-015-9515-5. Epub 2015 Nov 4. Mol Neurobiol. 2016. PMID: 26537901 Review. - Synaptic proteins in neuron-derived extracellular vesicles as biomarkers for Alzheimer's disease: novel methodology and clinical proof of concept.
Eitan E, Thornton-Wells T, Elgart K, Erden E, Gershun E, Levine A, Volpert O, Azadeh M, Smith DG, Kapogiannis D. Eitan E, et al. Extracell Vesicles Circ Nucl Acids. 2023 Mar;4(1):133-150. doi: 10.20517/evcna.2023.13. Epub 2023 Mar 31. Extracell Vesicles Circ Nucl Acids. 2023. PMID: 37842184 Free PMC article. - Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease.
Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D. Pulliam L, et al. J Neurovirol. 2019 Oct;25(5):702-709. doi: 10.1007/s13365-018-0695-4. Epub 2019 Jan 4. J Neurovirol. 2019. PMID: 30610738 Free PMC article. Review.
Cited by
- SARS-CoV-2 and Mitochondrial Proteins in Neural-Derived Exosomes of COVID-19.
Peluso MJ, Deeks SG, Mustapic M, Kapogiannis D, Henrich TJ, Lu S, Goldberg SA, Hoh R, Chen JY, Martinez EO, Kelly JD, Martin JN, Goetzl EJ. Peluso MJ, et al. Ann Neurol. 2022 Jun;91(6):772-781. doi: 10.1002/ana.26350. Epub 2022 Mar 30. Ann Neurol. 2022. PMID: 35285072 Free PMC article. - Mitochondrial dysfunction in brain tissues and Extracellular Vesicles Fragile X-associated tremor/ataxia syndrome.
Yao PJ, Manolopoulos A, Eren E, Rivera SM, Hessl DR, Hagerman R, Martinez-Cerdeno V, Tassone F, Kapogiannis D. Yao PJ, et al. Ann Clin Transl Neurol. 2024 Jun;11(6):1420-1429. doi: 10.1002/acn3.52040. Epub 2024 May 8. Ann Clin Transl Neurol. 2024. PMID: 38717724 Free PMC article. - Molecular Signatures of Mitochondrial Complexes Involved in Alzheimer's Disease via Oxidative Phosphorylation and Retrograde Endocannabinoid Signaling Pathways.
Chen F, Bai J, Zhong S, Zhang R, Zhang X, Xu Y, Zhao M, Zhao C, Zhou Z. Chen F, et al. Oxid Med Cell Longev. 2022 Apr 5;2022:9565545. doi: 10.1155/2022/9565545. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35432724 Free PMC article. - Mitochondrial Damage-Associated Molecular Patterns Content in Extracellular Vesicles Promotes Early Inflammation in Neurodegenerative Disorders.
Deus CM, Tavares H, Beatriz M, Mota S, Lopes C. Deus CM, et al. Cells. 2022 Aug 1;11(15):2364. doi: 10.3390/cells11152364. Cells. 2022. PMID: 35954208 Free PMC article. Review. - Alzheimer's Disease-Related Genes Identified by Linking Spatial Patterns of Pathology and Gene Expression.
Mullins R, Kapogiannis D. Mullins R, et al. Front Neurosci. 2022 Jun 14;16:908650. doi: 10.3389/fnins.2022.908650. eCollection 2022. Front Neurosci. 2022. PMID: 35774552 Free PMC article.
References
- Cao K., Dong Y.T., Xiang J., Xu Y., Hong W., Song H., Guan Z.Z. Reduced expression of SIRT1 and SOD-1 and the correlation between these levels in various regions of the brains of patients with Alzheimer’s disease. J. Clin. Pathol. 2018;71:1090–1099. doi: 10.1136/jclinpath-2018-205320. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous