Mycophenolate Mofetil after Rituximab for Childhood-Onset Complicated Frequently-Relapsing or Steroid-Dependent Nephrotic Syndrome - PubMed (original) (raw)
Randomized Controlled Trial
. 2022 Feb;33(2):401-419.
doi: 10.1681/ASN.2021050643. Epub 2021 Dec 8.
Mayumi Sako 3, Mari Oba 4, Seiji Tanaka 5, Riku Hamada 6, Tomoyuki Sakai 7, Yoko Ohwada 8, Takeshi Ninchoji 1, Tomohiko Yamamura 1, Hiroyuki Machida 9, Yuko Shima 10, Ryojiro Tanaka 11, Hiroshi Kaito 1 11, Yoshinori Araki 12, Tamaki Morohashi 13, Naonori Kumagai 14, Yoshimitsu Gotoh 15, Yohei Ikezumi 16, Takuo Kubota 17, Koichi Kamei 18, Naoya Fujita 19, Yasufumi Ohtsuka 20, Takayuki Okamoto 21, Takeshi Yamada 22, Eriko Tanaka 23, Masaki Shimizu 24, Tomoko Horinochi 1, Akihide Konishi 25, Takashi Omori 25, Koichi Nakanishi 26, Kenji Ishikura 27, Shuichi Ito 9, Hidefumi Nakamura 28, Kandai Nozu 1; Japanese Study Group of Kidney Disease in Children
Affiliations
- PMID: 34880074
- PMCID: PMC8819987
- DOI: 10.1681/ASN.2021050643
Randomized Controlled Trial
Mycophenolate Mofetil after Rituximab for Childhood-Onset Complicated Frequently-Relapsing or Steroid-Dependent Nephrotic Syndrome
Kazumoto Iijima et al. J Am Soc Nephrol. 2022 Feb.
Erratum in
- Correction: Mycophenolate Mofetil after Rituximab for Childhood-Onset Complicated Frequently-Relapsing or Steroid-Dependent Nephrotic Syndrome.
[No authors listed] [No authors listed] J Am Soc Nephrol. 2022 May;33(5):1050. doi: 10.1681/ASN.2022020134. J Am Soc Nephrol. 2022. PMID: 35487689 Free PMC article. No abstract available.
Abstract
Background: Rituximab is the standard therapy for childhood-onset complicated frequently relapsing or steroid-dependent nephrotic syndrome (FRNS/SDNS). However, most patients redevelop FRNS/SDNS after peripheral B cell recovery.
Methods: We conducted a multicenter, randomized, double-blind, placebo-controlled trial to examine whether mycophenolate mofetil (MMF) administration after rituximab can prevent treatment failure (FRNS, SDNS, steroid resistance, or use of immunosuppressive agents or rituximab). In total, 39 patients (per group) were treated with rituximab, followed by either MMF or placebo until day 505 (treatment period). The primary outcome was time to treatment failure (TTF) throughout the treatment and follow-up periods (until day 505 for the last enrolled patient).
Results: TTFs were clinically but not statistically significantly longer among patients given MMF after rituximab than among patients receiving rituximab monotherapy (median, 784.0 versus 472.5 days, hazard ratio [HR], 0.59; 95% confidence interval [95% CI], 0.34 to 1.05, log-rank test: _P_=0.07). Because most patients in the MMF group presented with treatment failure after MMF discontinuation, we performed a post-hoc analysis limited to the treatment period and found that MMF after rituximab prolonged the TTF and decreased the risk of treatment failure by 80% (HR, 0.20; 95% CI, 0.08 to 0.50). Moreover, MMF after rituximab reduced the relapse rate and daily steroid dose during the treatment period by 74% and 57%, respectively. The frequency and severity of adverse events were similar in both groups.
Conclusions: Administration of MMF after rituximab may sufficiently prevent the development of treatment failure and is well tolerated, although the relapse-preventing effect disappears after MMF discontinuation.
Keywords: childhood-onset; clinical trial; complicated frequently-relapsing/steroid-dependent nephrotic syndrome; mycophenolate mofetil; rituximab.
Copyright © 2022 by the American Society of Nephrology.
Figures
Graphical abstract
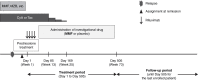
Figure 1.
Study design. After rituximab was administered at a dose of 375 mg/m2 (maximum dose: 500 mg) once weekly for 4 weeks, on days 1, 8, 15, and 22, MMF was administered at a dose of 1000–1200 mg/m2per day (maximum 2 g/day) twice daily after breakfast and dinner for 17 months (from day 29 until day 505). In the placebo group, rituximab was provided in the same manner, and placebo was administered instead of MMF. The follow-up period was defined as the time from day 506 to the last scheduled treatment date of the last enrolled patient. During the follow-up period, investigators followed-up all participants according to the specified schedule and conducted a follow-up survey using routine clinical data. MZB, mizoribine; Tac, tacrolimus.
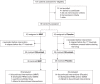
Figure 2.
Flow diagram.
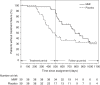
Figure 3.
Kaplan–Meier curves for treatment failure (frequent relapses, steroid dependence or resistance, or use of immunosuppressive agents or rituximab)-free survival. The times to treatment failure were not statistically significantly longer throughout the study period (the treatment period and follow-up period) among patients given MMF after rituximab than among patients receiving rituximab monotherapy (HR, 0.59; 95% CI, 0.34 to 1.05; P = 0.07) (see also Table 3a). However, during the treatment period, rituximab followed by MMF decreased the development of treatment failure by 80% compared with rituximab monotherapy (HR, 0.20; 95% CI, 0.08 to 0.50) (see also Table 3b).
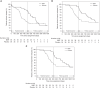
Figure 4.
Kaplan–Meier curves or cumulative incidence-free function for secondary outcomes. (A) Kaplan–Meier curves for relapse-free survival. (B) Cumulative incidence-free function for frequent relapses. (C) Cumulative incidence-free function for steroid-dependent relapses. Times to relapse and those to each of component outcomes of treatment failure such as frequent relapses and steroid dependent relapses were approximately 40% longer (although not statistically significantly) among patients given MMF after rituximab than among patients receiving rituximab monotherapy throughout the study (combined treatment and follow-up) period (HR, 95% CI; [A] 0.62, 0.30 to 1.04; [B] 0.56, 0.32 to 1.00; [C] 0.60, 0.34 to 1.08) (see also Tables 5a, 6a, and 7a). However, during the treatment period, MMF after rituximab decreased the occurrence of relapse, frequent relapses, and steroid-dependent relapses by 70%–80% compared with rituximab monotherapy (HR, 95% CI; [A] 0.28, 0.14 to 0.57; [B] 0.20, 0.08 to 0.48; [C] 0.22, 0.09 to 0.53) (see also Tables 5b, 6b, and 7b).
Figure 5.
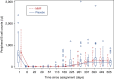
Peripheral B cell counts. Peripheral CD19-positive cell counts were monitored until day 505. Day 1 was the first day of rituximab administration. Boxes represent the quartile range for each grouping and time period, straight lines (beard) are connected from the top and bottom sides of the boxes to outliers within 1.5 times the quartile range width, and red crosses and blue open circles represent outlying observations in the MMF group and placebo group, respectively.
Similar articles
- Study protocol: mycophenolate mofetil as maintenance therapy after rituximab treatment for childhood-onset, complicated, frequently-relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicenter double-blind, randomized, placebo-controlled trial (JSKDC07).
Horinouchi T, Sako M, Nakanishi K, Ishikura K, Ito S, Nakamura H, Oba MS, Nozu K, Iijima K. Horinouchi T, et al. BMC Nephrol. 2018 Nov 1;19(1):302. doi: 10.1186/s12882-018-1099-7. BMC Nephrol. 2018. PMID: 30382824 Free PMC article. Clinical Trial. - Evaluation of mycophenolate mofetil or tacrolimus in children with steroid sensitive but frequently relapsing or steroid-dependent nephrotic syndrome.
Wang J, Mao J, Chen J, Fu H, Shen H, Zhu X, Liu A, Shu Q, Du L. Wang J, et al. Nephrology (Carlton). 2016 Jan;21(1):21-7. doi: 10.1111/nep.12537. Nephrology (Carlton). 2016. PMID: 26697959 - Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial.
Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, Miura K, Aya K, Nakanishi K, Ohtomo Y, Takahashi S, Tanaka R, Kaito H, Nakamura H, Ishikura K, Ito S, Ohashi Y; Rituximab for Childhood-onset Refractory Nephrotic Syndrome (RCRNS) Study Group. Iijima K, et al. Lancet. 2014 Oct 4;384(9950):1273-81. doi: 10.1016/S0140-6736(14)60541-9. Epub 2014 Jun 22. Lancet. 2014. PMID: 24965823 Clinical Trial. - Rituximab for nephrotic syndrome in children.
Iijima K, Sako M, Nozu K. Iijima K, et al. Clin Exp Nephrol. 2017 Apr;21(2):193-202. doi: 10.1007/s10157-016-1313-5. Epub 2016 Jul 15. Clin Exp Nephrol. 2017. PMID: 27422620 Free PMC article. Review. - Rituximab in steroid-sensitive nephrotic syndrome: lessons from clinical trials.
Iijima K, Sako M, Kamei K, Nozu K. Iijima K, et al. Pediatr Nephrol. 2018 Sep;33(9):1449-1455. doi: 10.1007/s00467-017-3746-9. Epub 2017 Jul 17. Pediatr Nephrol. 2018. PMID: 28717938 Free PMC article. Review.
Cited by
- Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children.
Larkins NG, Hahn D, Liu ID, Willis NS, Craig JC, Hodson EM. Larkins NG, et al. Cochrane Database Syst Rev. 2024 Nov 8;11(11):CD002290. doi: 10.1002/14651858.CD002290.pub6. Cochrane Database Syst Rev. 2024. PMID: 39513526 Review. - Expression of SIRT6 and VNN1 in children with primary nephrotic syndrome and their correlation with acute kidney injury.
Hao F, Zhang S, Gao Y. Hao F, et al. Am J Transl Res. 2024 Sep 15;16(9):5122-5129. doi: 10.62347/TOUH6376. eCollection 2024. Am J Transl Res. 2024. PMID: 39398594 Free PMC article. - The efficacy and safety of rituximab with or without glucocorticoid in inducing remission of MCD with different clinical presentations in adults: a retrospective study.
Sun Y, Li Z, Sun J, Zhang S, Wang R, Chen B. Sun Y, et al. Clin Kidney J. 2024 May 3;17(6):sfae139. doi: 10.1093/ckj/sfae139. eCollection 2024 Jun. Clin Kidney J. 2024. PMID: 38854425 Free PMC article. - Japanese clinical practice patterns of rituximab treatment for minimal change disease in adults 2021: A web-based questionnaire survey of certified nephrologists.
Koizumi M, Ishimoto T, Shimizu S, Sasaki S, Kurita N, Wada T. Koizumi M, et al. PLoS One. 2024 Mar 29;19(3):e0299053. doi: 10.1371/journal.pone.0299053. eCollection 2024. PLoS One. 2024. PMID: 38551948 Free PMC article.
References
- International Study of Kidney Disease in Children : Nephrotic syndrome in children: Prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int 13: 159–165, 1978 - PubMed
- Kikunaga K, Ishikura K, Terano C, Sato M, Komaki F, Hamasaki Y, et al. ; Japanese Pediatric Survey Holding Information of NEphrotic syndrome (JP-SHINE) study of the Japanese Study Group of Renal Disease in Children : High incidence of idiopathic nephrotic syndrome in East Asian children: a nationwide survey in Japan (JP-SHINE study). Clin Exp Nephrol 21: 651–657, 2017 - PubMed
- International Study of Kidney Disease in Children : The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr 98: 561–564, 1981 - PubMed
- Noone DG, Iijima K, Parekh R: Idiopathic nephrotic syndrome in children. Lancet 392: 61–74, 2018 - PubMed
- Kidney Disease Improving Global Outcomes (KDIGO): Clinical Practice Guideline for the Management of Glomerular Diseases: Chapter 4: Nephrotic syndrome in children. Available at: https://kdigo.org/wp-content/uploads/2017/02/KDIGO-Glomerular-Diseases-G.... Accessed May 1, 2021
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources