Ambulatory Fluoroquinolone Use in the United States, 2015-2019 - PubMed (original) (raw)
Ambulatory Fluoroquinolone Use in the United States, 2015-2019
Siddhi Pramod Umarje et al. Open Forum Infect Dis. 2021.
Abstract
Background: Frequently used fluoroquinolones have been subject to increasing safety concerns and regulatory alerts. This study characterized ambulatory fluoroquinolone utilization in the United States and evaluated the impact of 2016 Food and Drug Administration (FDA) safety advisories on its use.
Methods: We used IQVIA's National Disease and Therapeutic Index to quantify adult outpatient fluoroquinolone use ("treatment visits"). Descriptive statistics and segmented regression were used to report trends and quantify the varied use before and after FDA's 2016 alerts.
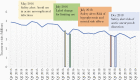
Results: Between 2015 to 2019, fluoroquinolone use decreased by 26.7% (18.7 million treatment visits in 2015 to 13.7 million treatment visits in 2019). Annual use declined by 44%, 24%, and 24% for respiratory, urogenital, and gastrointestinal conditions, respectively; and by 66% among providers ≤44 years old vs negligible decline among those ≥65 years old. Before 2016 FDA advisories, there were approximately 4.8 million fluoroquinolone treatment visits/quarter, which had a statistically significant immediate drop by 641035 visits (95% confidence interval [CI], -937368 to -344702; _P_=.000) after FDA's 2016 advisories. A statistically significant difference of approximately 45000 visits/quarter (95% CI, -85956 to -3122; _P_=.036) was observed after the advisories.
Conclusions: Large reductions in ambulatory fluoroquinolone use in the United States have coincided with increasing evidence of safety concerns and FDA advisories. However, fluoroquinolone use varies significantly based on patient and provider characteristics, suggesting heterogeneous effects of emerging risks on clinical practice.
Keywords: antibiotic stewardship; black-box warnings; fluoroquinolones; segmented regression; trends.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2021.
Figures
Figure 1.
Per-quarter utilization of fluoroquinolone antibiotics.
Similar articles
- Association of Fluoroquinolone Prescribing Rates With Black Box Warnings from the US Food and Drug Administration.
Sankar A, Swanson KM, Zhou J, Jena AB, Ross JS, Shah ND, Karaca-Mandic P. Sankar A, et al. JAMA Netw Open. 2021 Dec 1;4(12):e2136662. doi: 10.1001/jamanetworkopen.2021.36662. JAMA Netw Open. 2021. PMID: 34851398 Free PMC article. - Current prescribing practices and guideline concordance for the treatment of uncomplicated urinary tract infections in women.
Langner JL, Chiang KF, Stafford RS. Langner JL, et al. Am J Obstet Gynecol. 2021 Sep;225(3):272.e1-272.e11. doi: 10.1016/j.ajog.2021.04.218. Epub 2021 Apr 20. Am J Obstet Gynecol. 2021. PMID: 33848538 - Outpatient Fluoroquinolone Prescription Fills in the United States, 2014 to 2020: Assessing the Impact of Food and Drug Administration Safety Warnings.
Buehrle DJ, Wagener MM, Clancy CJ. Buehrle DJ, et al. Antimicrob Agents Chemother. 2021 Jun 17;65(7):e0015121. doi: 10.1128/AAC.00151-21. Epub 2021 Jun 17. Antimicrob Agents Chemother. 2021. PMID: 33875430 Free PMC article. - Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1997.
Schappert SM. Schappert SM. Vital Health Stat 13. 1999 Nov;(143):i-iv, 1-39. Vital Health Stat 13. 1999. PMID: 10633576 Review. - Fluoroquinolone-Associated Tendinopathy: Does Levofloxacin Pose the Greatest Risk?
Bidell MR, Lodise TP. Bidell MR, et al. Pharmacotherapy. 2016 Jun;36(6):679-93. doi: 10.1002/phar.1761. Epub 2016 Jun 11. Pharmacotherapy. 2016. PMID: 27138564 Review.
Cited by
- The burden of antimicrobial resistance in the Americas in 2019: a cross-country systematic analysis.
Antimicrobial Resistance Collaborators. Antimicrobial Resistance Collaborators. Lancet Reg Health Am. 2023 Aug 8;25:100561. doi: 10.1016/j.lana.2023.100561. eCollection 2023 Sep. Lancet Reg Health Am. 2023. PMID: 37727594 Free PMC article. - Impact of Fluoroquinolone Susceptibility Suppression on Discharge Prescribing for Acute Uncomplicated Cystitis.
Hayden DA, White BP, Neely S, Bennett KK. Hayden DA, et al. Open Forum Infect Dis. 2023 Sep 8;10(10):ofad459. doi: 10.1093/ofid/ofad459. eCollection 2023 Oct. Open Forum Infect Dis. 2023. PMID: 37849508 Free PMC article. - Do fluoroquinolones increase aortic aneurysm or dissection incidence and mortality? A systematic review and meta-analysis.
Chen C, Patterson B, Simpson R, Li Y, Chen Z, Lv Q, Guo D, Li X, Fu W, Guo B. Chen C, et al. Front Cardiovasc Med. 2022 Aug 9;9:949538. doi: 10.3389/fcvm.2022.949538. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36017083 Free PMC article. - Advantages of analysing both pairwise SNV-distance and differing SNVs between Mycobacterium tuberculosis isolates for recurrent tuberculosis cause determination.
Sadovska D, Nodieva A, Pole I, Ķimsis J, Vīksna A, Ozere I, Norvaiša I, Bandere D, Ranka R. Sadovska D, et al. Microb Genom. 2023 Mar;9(3):mgen000956. doi: 10.1099/mgen.0.000956. Microb Genom. 2023. PMID: 36951900 Free PMC article.
References
- US Food and Drug Administration. FDA-approved drugs. https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed 23 November 2019.
- Oliphant CM, Green GM.. Quinolones: a comprehensive review. Am Fam Physician 2002; 65:455–64. - PubMed
- Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States, annual report 2016. https://www.cdc.gov/antibiotic-use/community/programs-measurement/state-.... Accessed 23 November 2019.
- Bailey RR, Kirk JA, Peddie BA.. Norfloxacin-induced rheumatic disease. N Z Med J 1983; 96:590. - PubMed