Effectiveness of Fosfomycin for the Treatment of Multidrug-Resistant Escherichia coli Bacteremic Urinary Tract Infections: A Randomized Clinical Trial - PubMed (original) (raw)
Randomized Controlled Trial
. 2022 Jan 4;5(1):e2137277.
doi: 10.1001/jamanetworkopen.2021.37277.
Inmaculada López-Hernández 1, Clara Rosso-Fernandez 2, Isabel M Morales 3, Zaira R Palacios-Baena 1, Alicia Hernández-Torres 4, Esperanza Merino de Lucas 5, Laura Escolà-Vergé 6, Elena Bereciartua 7, Elisa García-Vázquez 4, Vicente Pintado 8, Lucía Boix-Palop 9, Clara Natera-Kindelán 10, Luisa Sorlí 11, Nuria Borrell 12, Livia Giner-Oncina 5, Concha Amador-Prous 13, Evelyn Shaw 14, Alfredo Jover-Saenz 15, Jose Molina 16, Rosa M Martínez-Alvarez 17 18, Carlos J Dueñas 19 20, Jorge Calvo-Montes 21, Jose T Silva 22, Miguel A Cárdenes 23, María Lecuona 24, Virginia Pomar 25, Lucía Valiente de Santis 26, Genoveva Yagüe-Guirao 27, María Angeles Lobo-Acosta 2, Vicente Merino-Bohórquez 28, Alvaro Pascual 1, Jesús Rodríguez-Baño 1; REIPI-GEIRAS-FOREST group
Collaborators, Affiliations
- PMID: 35024838
- PMCID: PMC8759008
- DOI: 10.1001/jamanetworkopen.2021.37277
Randomized Controlled Trial
Effectiveness of Fosfomycin for the Treatment of Multidrug-Resistant Escherichia coli Bacteremic Urinary Tract Infections: A Randomized Clinical Trial
Jesús Sojo-Dorado et al. JAMA Netw Open. 2022.
Abstract
Importance: The consumption of broad-spectrum drugs has increased as a consequence of the spread of multidrug-resistant (MDR) Escherichia coli. Finding alternatives for these infections is critical, for which some neglected drugs may be an option.
Objective: To determine whether fosfomycin is noninferior to ceftriaxone or meropenem in the targeted treatment of bacteremic urinary tract infections (bUTIs) due to MDR E coli.
Design, setting, and participants: This multicenter, randomized, pragmatic, open clinical trial was conducted at 22 Spanish hospitals from June 2014 to December 2018. Eligible participants were adult patients with bacteremic urinary tract infections due to MDR E coli; 161 of 1578 screened patients were randomized and followed up for 60 days. Data were analyzed in May 2021.
Interventions: Patients were randomized 1 to 1 to receive intravenous fosfomycin disodium at 4 g every 6 hours (70 participants) or a comparator (ceftriaxone or meropenem if resistant; 73 participants) with the option to switch to oral fosfomycin trometamol for the fosfomycin group or an active oral drug or parenteral ertapenem for the comparator group after 4 days.
Main outcomes and measures: The primary outcome was clinical and microbiological cure (CMC) 5 to 7 days after finalization of treatment; a noninferiority margin of 7% was considered.
Results: Among 143 patients in the modified intention-to-treat population (median [IQR] age, 72 [62-81] years; 73 [51.0%] women), 48 of 70 patients (68.6%) treated with fosfomycin and 57 of 73 patients (78.1%) treated with comparators reached CMC (risk difference, -9.4 percentage points; 1-sided 95% CI, -21.5 to ∞ percentage points; P = .10). While clinical or microbiological failure occurred among 10 patients (14.3%) treated with fosfomycin and 14 patients (19.7%) treated with comparators (risk difference, -5.4 percentage points; 1-sided 95% CI, -∞ to 4.9; percentage points; P = .19), an increased rate of adverse event-related discontinuations occurred with fosfomycin vs comparators (6 discontinuations [8.5%] vs 0 discontinuations; P = .006). In an exploratory analysis among a subset of 38 patients who underwent rectal colonization studies, patients treated with fosfomycin acquired a new ceftriaxone-resistant or meropenem-resistant gram-negative bacteria at a decreased rate compared with patients treated with comparators (0 of 21 patients vs 4 of 17 patients [23.5%]; 1-sided P = .01).
Conclusions and relevance: This study found that fosfomycin did not demonstrate noninferiority to comparators as targeted treatment of bUTI from MDR E coli; this was due to an increased rate of adverse event-related discontinuations. This finding suggests that fosfomycin may be considered for selected patients with these infections.
Trial registration: ClinicalTrials.gov Identifier: NCT02142751.
Conflict of interest statement
Conflict of Interest Disclosures: Dr Merino de Lucas reported receiving grants from Merck Sharp and Dohme (MSD) outside the submitted work. Dr Pintado reported receiving personal fees from Shionogi, Pfizer, MSD, and Correvio outside the submitted work. Dr Boix-Palop reported receiving travel and congress registration expenses from Pizer and Menarini outside the submitted work. Dr Calvo-Montes reported receiving grants from MSD and Pfizer outside the submitted work. Dr Lecuona reported receiving grants from BioMérieux and Mapfre outside the submitted work. Dr Merino-Bohórquez reported receiving personal fees from AbbVie and Pfizer and grants from Kern Pharma and Amgen outside the submitted work. No other disclosures were reported.
Figures
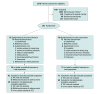
Figure.. Patient Recruitment and Flow Through Study
TOC indicates test of cure.
Comment in
- The Role of Intravenous Fosfomycin: Finding Our Way Out of Dante's Forest Dark.
Tamma PD, Bonomo RA, Stiefel U. Tamma PD, et al. JAMA Netw Open. 2022 Jan 4;5(1):e2138691. doi: 10.1001/jamanetworkopen.2021.38691. JAMA Netw Open. 2022. PMID: 35024841 No abstract available.
Similar articles
- Effectiveness of fosfomycin trometamol as oral step-down therapy for bacteraemic urinary tract infections due to MDR Escherichia coli: a post hoc analysis of the FOREST randomized trial.
Sojo-Dorado J, López-Hernández I, Hernández-Torres A, Retamar-Gentil P, Merino de Lucas E, Escolà-Vergé L, Bereciartua E, García-Vázquez E, Pintado V, Boix-Palop L, Natera-Kindelán C, Sorlí L, Borrell N, Amador-Prous C, Shaw E, Jover-Saenz A, Molina J, Martínez-Álvarez RM, Dueñas CJ, Calvo-Montes J, Lecuona M, Pomar V, Borreguero I, Palomo-Jiménez V, Docobo-Pérez F, Pascual Á, Rodríguez-Baño J. Sojo-Dorado J, et al. J Antimicrob Chemother. 2023 Jul 5;78(7):1658-1666. doi: 10.1093/jac/dkad147. J Antimicrob Chemother. 2023. PMID: 37260299 Free PMC article. Clinical Trial. - Fosfomycin versus meropenem in bacteraemic urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli (FOREST): study protocol for an investigator-driven randomised controlled trial.
Rosso-Fernández C, Sojo-Dorado J, Barriga A, Lavín-Alconero L, Palacios Z, López-Hernández I, Merino V, Camean M, Pascual A, Rodríguez-Baño J; FOREST Study Group. Rosso-Fernández C, et al. BMJ Open. 2015 Mar 31;5(3):e007363. doi: 10.1136/bmjopen-2014-007363. BMJ Open. 2015. PMID: 25829373 Free PMC article. Clinical Trial. - Effect of Piperacillin-Tazobactam vs Meropenem on 30-Day Mortality for Patients With E coli or Klebsiella pneumoniae Bloodstream Infection and Ceftriaxone Resistance: A Randomized Clinical Trial.
Harris PNA, Tambyah PA, Lye DC, Mo Y, Lee TH, Yilmaz M, Alenazi TH, Arabi Y, Falcone M, Bassetti M, Righi E, Rogers BA, Kanj S, Bhally H, Iredell J, Mendelson M, Boyles TH, Looke D, Miyakis S, Walls G, Al Khamis M, Zikri A, Crowe A, Ingram P, Daneman N, Griffin P, Athan E, Lorenc P, Baker P, Roberts L, Beatson SA, Peleg AY, Harris-Brown T, Paterson DL; MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN). Harris PNA, et al. JAMA. 2018 Sep 11;320(10):984-994. doi: 10.1001/jama.2018.12163. JAMA. 2018. PMID: 30208454 Free PMC article. Clinical Trial. - The role of fosfomycin for multidrug-resistant gram-negative infections.
Bassetti M, Graziano E, Berruti M, Giacobbe DR. Bassetti M, et al. Curr Opin Infect Dis. 2019 Dec;32(6):617-625. doi: 10.1097/QCO.0000000000000597. Curr Opin Infect Dis. 2019. PMID: 31567411 Review. - Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.
Zhanel GG, Golden AR, Zelenitsky S, Wiebe K, Lawrence CK, Adam HJ, Idowu T, Domalaon R, Schweizer F, Zhanel MA, Lagacé-Wiens PRS, Walkty AJ, Noreddin A, Lynch Iii JP, Karlowsky JA. Zhanel GG, et al. Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review.
Cited by
- A multicentre study to determine the in vitro efficacy of flomoxef against extended-spectrum beta-lactamase producing Escherichia coli in Malaysia.
Yap PSX, Chong CW, Ponnampalavanar S, Ramli R, Harun A, Tengku Jamaluddin TZM, Ahmed Khan A, Ngoi ST, Lee YQ, Lau MY, Tan SC, Kong ZX, Woon JJ, Mak ST, Abdul Jabar K, Karunakaran R, Ismail Z, Salleh SA, Md Noor SS, Masri SN, Mohd Taib N, Jasni AS, Tee LH, Leong KC, Lim VKE, Abu Bakar S, Teh CSJ. Yap PSX, et al. PeerJ. 2023 Nov 28;11:e16393. doi: 10.7717/peerj.16393. eCollection 2023. PeerJ. 2023. PMID: 38047021 Free PMC article. - Extended-spectrum β-lactamase-producing and carbapenem-resistant Enterobacterales bloodstream infection after solid organ transplantation: Recent trends in epidemiology and therapeutic approaches.
Pérez-Nadales E, Fernández-Ruiz M, Gutiérrez-Gutiérrez B, Pascual Á, Rodríguez-Baño J, Martínez-Martínez L, Aguado JM, Torre-Cisneros J. Pérez-Nadales E, et al. Transpl Infect Dis. 2022 Aug;24(4):e13881. doi: 10.1111/tid.13881. Epub 2022 Jun 28. Transpl Infect Dis. 2022. PMID: 35691028 Free PMC article. Review. - Comparison of three doses of amikacin on alternate days with a daily dose of meropenem during the same period for the treatment of urinary tract infection with E. coli: a double-blind clinical trial.
Mohsenpour B, Ahmadi A, Azizzadeh H, Ghaderi E, Hajibagheri K, Afrasiabian S, Lotfi G, Farzinpoor Z. Mohsenpour B, et al. BMC Res Notes. 2024 Jan 25;17(1):38. doi: 10.1186/s13104-023-06654-y. BMC Res Notes. 2024. PMID: 38273327 Free PMC article. Clinical Trial. - Longitudinal analysis within one hospital in sub-Saharan Africa over 20 years reveals repeated replacements of dominant clones of Klebsiella pneumoniae and stresses the importance to include temporal patterns for vaccine design considerations.
Heinz E, Pearse O, Zuza A, Bilima S, Msefula C, Musicha P, Siyabu P, Tewesa E, Graf FE, Lester R, Lissauer S, Cornick J, Lewis JM, Kawaza K, Thomson NR, Feasey NA. Heinz E, et al. Genome Med. 2024 May 6;16(1):67. doi: 10.1186/s13073-024-01342-3. Genome Med. 2024. PMID: 38711148 Free PMC article. - Urinary Tract Infections: The Current Scenario and Future Prospects.
Mancuso G, Midiri A, Gerace E, Marra M, Zummo S, Biondo C. Mancuso G, et al. Pathogens. 2023 Apr 20;12(4):623. doi: 10.3390/pathogens12040623. Pathogens. 2023. PMID: 37111509 Free PMC article. Review.
References
- Holland MS, Nobrega D, Peirano G, Naugler C, Church DL, Pitout JDD. Molecular epidemiology of Escherichia coli causing bloodstream infections in a centralized Canadian region: a population-based surveillance study. Clin Microbiol Infect. 2020;26(11):1554.e1-1554.e8. doi:10.1016/j.cmi.2020.02.019 - DOI - PubMed