Nuclear Fragility in Radiation-Induced Senescence: Blebs and Tubes Visualized by 3D Electron Microscopy - PubMed (original) (raw)
Nuclear Fragility in Radiation-Induced Senescence: Blebs and Tubes Visualized by 3D Electron Microscopy
Benjamin M Freyter et al. Cells. 2022.
Abstract
Irreparable DNA damage following ionizing radiation (IR) triggers prolonged DNA damage response and induces premature senescence. Cellular senescence is a permanent state of cell-cycle arrest characterized by chromatin restructuring, altered nuclear morphology and acquisition of secretory phenotype, which contributes to senescence-related inflammation. However, the mechanistic connections for radiation-induced DNA damage that trigger these senescence-associated hallmarks are poorly understood. In our in vitro model of radiation-induced senescence, mass spectrometry-based proteomics was combined with high-resolution imaging techniques to investigate the interrelations between altered chromatin compaction, nuclear envelope destabilization and nucleo-cytoplasmic chromatin blebbing. Our findings confirm the general pathophysiology of the senescence-response, with disruption of nuclear lamin organization leading to extensive chromatin restructuring and destabilization of the nuclear membrane with release of chromatin fragments into the cytosol, thereby activating cGAS-STING-dependent interferon signaling. By serial block-face scanning electron microscopy (SBF-SEM) whole-cell datasets were acquired to investigate the morphological organization of senescent fibroblasts. High-resolution 3-dimensional (3D) reconstruction of the complex nuclear shape allows us to precisely visualize the segregation of nuclear blebs from the main nucleus and their fusion with lysosomes. By multi-view 3D electron microscopy, we identified nanotubular channels formed in lamin-perturbed nuclei of senescent fibroblasts; the potential role of these nucleo-cytoplasmic nanotubes for expulsion of damaged chromatin has to be examined.
Keywords: cGAS-STING signaling; cellular senescence; chromatin reorganization; cytosolic chromatin fragments (CCF); ionizing radiation; nuclear blebbing; radiation-induced senescence; serial block-face scanning electron microscopy (SBF-SEM); transmission electron microscopy (TEM).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Figure 1
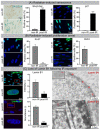
Cellular senescence following IR. (A) Increased numbers of SA-β-Gal-positive and p21-positive cells following IR. (B) Decrease in Ki-67-positive and BrdU-positive cells. (C) Lamin B1 loss in nuclear envelope following IR exposure visualized by IFM (left) and TEM (right). Quantification of lamin B1 in WI-38 fibroblasts by IFM (top middle), and MS (bottom middle). Data are presented as mean ± SEM, * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 2
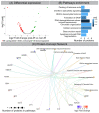
Radiation-induced changes in protein expression. (A) Volcano plot showing differential protein expression following IR. (B) Senescence-related pathway enrichment results were generated using ReactomePA R package. (C) Visualization of enriched pathways components by enrichplot R package.
Figure 3
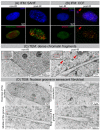
IR-induced morphological changes in WI-38 fibroblasts. (A) Visualization of SAHF formation by IFM. (B) Visualization of CCF formation by IFM. (C) Visualization of dense chromatin fragments (marked by red arrows) by TEM. (D) Visualization of nuclear groove in senescent fibroblast by TEM.
Figure 4
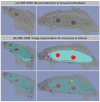
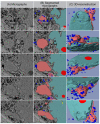
SBF-SEM: 3D reconstruction of senescent fibroblast. (A) Original SBF-SEM sections were used for 3D reconstruction. (B) Serial SBF-SEM sections were segmented for structures of interest: nucleus (light-blue), nucleoli (red), lysosomes (blue), and CCF (light-red), nanotubes (yellow), cytosol (gray) and used for 3D visualization (bottom row: transparent view).
Figure 5
SBF-SEM: Separation process of CCF. (A) Original micrographs presenting detaching CCF. (B) Segmented micrographs of the same regions. (C) 3D reconstruction of detaching CCF: nucleus (light-blue), nucleoli (red), lysosomes (blue), and CCF (light-red), nanotubes (yellow).
Figure 6
Nucleo-cytoplasmic nanotube. (A) Original micrographs showing cross-sections of the nanotube. (B) Segmented micrographs for the same area: nucleus (light-blue), nucleoli (red), nanotubes (yellow). (C) Models showing nanotube’s appearance from within the nucleus (1st and 2nd image from top), segmented volume showing the nanotube from side (3rd and 4th image from top), and original micrograph of the same region. (D) Visualization of the nanotube’s interior.
Similar articles
- Histone Variant H2A.J Marks Persistent DNA Damage and Triggers the Secretory Phenotype in Radiation-Induced Senescence.
Isermann A, Mann C, Rübe CE. Isermann A, et al. Int J Mol Sci. 2020 Nov 30;21(23):9130. doi: 10.3390/ijms21239130. Int J Mol Sci. 2020. PMID: 33266246 Free PMC article. - Mitochondria-to-nucleus retrograde signaling drives formation of cytoplasmic chromatin and inflammation in senescence.
Vizioli MG, Liu T, Miller KN, Robertson NA, Gilroy K, Lagnado AB, Perez-Garcia A, Kiourtis C, Dasgupta N, Lei X, Kruger PJ, Nixon C, Clark W, Jurk D, Bird TG, Passos JF, Berger SL, Dou Z, Adams PD. Vizioli MG, et al. Genes Dev. 2020 Mar 1;34(5-6):428-445. doi: 10.1101/gad.331272.119. Epub 2020 Jan 30. Genes Dev. 2020. PMID: 32001510 Free PMC article. - Olive phenols preserve lamin B1 expression reducing cGAS/STING/NFκB-mediated SASP in ionizing radiation-induced senescence.
Frediani E, Scavone F, Laurenzana A, Chillà A, Tortora K, Cimmino I, Leri M, Bucciantini M, Mangoni M, Fibbi G, Del Rosso M, Mocali A, Giovannelli L, Margheri F. Frediani E, et al. J Cell Mol Med. 2022 Apr;26(8):2337-2350. doi: 10.1111/jcmm.17255. Epub 2022 Mar 12. J Cell Mol Med. 2022. PMID: 35278036 Free PMC article. - The functional impact of nuclear reorganization in cellular senescence.
Rocha A, Dalgarno A, Neretti N. Rocha A, et al. Brief Funct Genomics. 2022 Jan 25;21(1):24-34. doi: 10.1093/bfgp/elab012. Brief Funct Genomics. 2022. PMID: 33755107 Free PMC article. Review. - Cellular senescence and senescence-associated secretory phenotype via the cGAS-STING signaling pathway in cancer.
Loo TM, Miyata K, Tanaka Y, Takahashi A. Loo TM, et al. Cancer Sci. 2020 Feb;111(2):304-311. doi: 10.1111/cas.14266. Epub 2019 Dec 27. Cancer Sci. 2020. PMID: 31799772 Free PMC article. Review.
Cited by
- The Chromosome Passenger Complex (CPC) Components and Its Associated Pathways Are Promising Candidates to Differentiate Between Normosensitive and Radiosensitive ATM-Mutated Cells.
Dietz A, Subedi P, Azimzadeh O, Duchrow L, Kaestle F, Paetzold J, Katharina Payer S, Hornhardt S, von Toerne C, Hauck SM, Kempkes B, Kuklik-Roos C, Brandes D, Borkhardt A, Moertl S, Gomolka M. Dietz A, et al. Biomark Insights. 2024 Oct 30;19:11772719241274017. doi: 10.1177/11772719241274017. eCollection 2024. Biomark Insights. 2024. PMID: 39493730 Free PMC article. - STING signaling in inflammaging: a new target against musculoskeletal diseases.
Song C, Hu Z, Xu D, Bian H, Lv J, Zhu X, Zhang Q, Su L, Yin H, Lu T, Li Y. Song C, et al. Front Immunol. 2023 Jul 10;14:1227364. doi: 10.3389/fimmu.2023.1227364. eCollection 2023. Front Immunol. 2023. PMID: 37492580 Free PMC article. Review. - Towards unravelling biological mechanisms behind radiation-induced oral mucositis via mass spectrometry-based proteomics.
Subedi P, Huber K, Sterr C, Dietz A, Strasser L, Kaestle F, Hauck SM, Duchrow L, Aldrian C, Monroy Ordonez EB, Luka B, Thomsen AR, Henke M, Gomolka M, Rößler U, Azimzadeh O, Moertl S, Hornhardt S. Subedi P, et al. Front Oncol. 2023 Jun 13;13:1180642. doi: 10.3389/fonc.2023.1180642. eCollection 2023. Front Oncol. 2023. PMID: 37384298 Free PMC article. - Connecting the dots: Neuronal senescence, stress granules, and neurodegeneration.
Ma Y, Farny NG. Ma Y, et al. Gene. 2023 Jun 30;871:147437. doi: 10.1016/j.gene.2023.147437. Epub 2023 Apr 20. Gene. 2023. PMID: 37084987 Free PMC article. Review. - Clinical Trial in a Dish for Space Radiation Countermeasure Discovery.
Cao X, Weil MM, Wu JC. Cao X, et al. Life Sci Space Res (Amst). 2022 Nov;35:140-149. doi: 10.1016/j.lssr.2022.05.006. Epub 2022 May 27. Life Sci Space Res (Amst). 2022. PMID: 36336359 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials