Machine Learning and In Vitro Chemical Screening of Potential α-Amylase and α-Glucosidase Inhibitors from Thai Indigenous Plants - PubMed (original) (raw)
Machine Learning and In Vitro Chemical Screening of Potential α-Amylase and α-Glucosidase Inhibitors from Thai Indigenous Plants
Tarapong Srisongkram et al. Nutrients. 2022.
Abstract
Diabetes mellitus is a major predisposing factor for cardiovascular disease and mortality. α-Amylase and α-glucosidase enzymes are the rate-limiting steps for carbohydrate digestion. The inhibition of these two enzymes is clinically used for the treatment of diabetes mellitus. Here, in vitro study and machine learning models were employed for the chemical screening of inhibiting the activity of 31 plant samples on α-amylase and α-glucosidase enzymes. The results showed that the ethanolic twig extract of Pinus kesiya had the highest inhibitory activity against the α-amylase enzyme. The respective ethanolic extract of Croton oblongifolius stem, Parinari anamense twig, and Polyalthia evecta leaf showed high inhibitory activity against the α-glucosidase enzyme. The classification analysis revealed that the α-glucosidase inhibitory activity of Thai indigenous plants was more predictive based on phytochemical constituents, compared with the α-amylase inhibitory activity (1.00 versus 0.97 accuracy score). The correlation loading plot revealed that flavonoids and alkaloids contributed to the α-amylase inhibitory activity, while flavonoids, tannins, and reducing sugars contributed to the α-glucosidase inhibitory activity. In conclusion, the ethanolic extracts of P. kesiya, C. oblongifolius, P. anamense, and P. evecta have the potential for further chemical characterization and the development of anti-diabetic recipes.
Keywords: Thai indigenous plants; anti-hyperglycemic; classification analysis; in vitro screening; machine learning; multivariate analysis; reinforcement learning; α-amylase; α-glucosidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
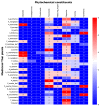
Figure 1
Phytochemical constituents of 31 TTM plant samples. The color bar of blue color to red color represents the amount, from not present (0 scores) to the highest presence (5 scores) of the phytochemical constituents in each sample.
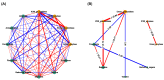
Figure 2
Correlation-based network analysis between α-amylase and α-glucosidase inhibitory activities and phytochemical components of 31 TTM plants samples. (A) Spearman’s correlation analysis between α-amylase and α-glucosidase inhibitory activities (i.e., Emax and IC50 against α-amylase and α-glucosidase enzymes) and eight phytochemical constituents found in 31 TTM samples. (B) Significance Spearman’s correlation between the α-amylase and α-glucosidase inhibitory activities and phytochemical compounds of 31 medicinal Thai herb samples (p < 0.05). The IC50 was categorized as active: 1 (IC50 can be calculated), and inactive: 0 (IC50 cannot be calculated). Each green node and orange node determines the phytocomponents, and bioactivities of the TTM, respectively. The respective positive (+) and negative (−) correlations (r) are presented in light-blue and red edges (connection line between two nodes). The thickness of each edge represents Spearman’s correlation coefficient (r).
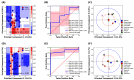
Figure 3
Classification analysis based on the random forest model of 31 TTM plant samples against α-amylase and α-glucosidase enzymes. (A) Decision boundary and score plot between principal component 1 (PC1) and component 5 (PC5) to predict the α-amylase inhibitory activity. Blue and red surfaces or dots indicate the inactive and active α-amylase inhibitory activity. (B) Receiving operating characteristic (ROC) of 5-fold cross-validation on the prediction of amylase inhibitory activity. (C) Loading plot of principal component analysis between PC1 to PC5. The blue solid circle indicates the correlation of the component in PC1 and PC5 at 1.0, and the red dash circle indicates the correlation of the component in PC1 and PC5 at 0.5. (D) Decision surface and score plot between principal component 3 (PC3) and component 4 (PC4) to predict the α-glucosidase inhibitory activity. Blue and red surfaces or dots indicate the inactive and active α-glucosidase inhibitory activity. (E) Receiving operating characteristic (ROC) of 5-fold cross-validation on the prediction of α-glucosidase inhibitory activity. (F) Loading plot of principal component analysis between PC3 to PC4. The blue solid circle indicates the correlation of the component in PC3 and PC4 at 1.0, whereas the red dash circle indicates the correlation of the component in PC3 and PC4 at 0.5.
Similar articles
- Evaluation of In Vitro α-Amylase and α-Glucosidase Inhibitory Potentials of 14 Medicinal Plants Constituted in Thai Folk Antidiabetic Formularies.
Somtimuang C, Olatunji OJ, Ovatlarnporn C. Somtimuang C, et al. Chem Biodivers. 2018 Apr;15(4):e1800025. doi: 10.1002/cbdv.201800025. Epub 2018 Apr 16. Chem Biodivers. 2018. PMID: 29460340 - Inhibitory effect of Azadirachta indica A. juss leaf extract on the activities of alpha-amylase and alpha-glucosidase.
Kazeem MI, Dansu TV, Adeola SA. Kazeem MI, et al. Pak J Biol Sci. 2013 Nov 1;16(21):1358-62. doi: 10.3923/pjbs.2013.1358.1362. Pak J Biol Sci. 2013. PMID: 24511747 - Antioxidative activity and inhibition of key enzymes linked to type-2 diabetes (α-glucosidase and α-amylase) by Khaya senegalensis.
Ibrahim MA, Koorbanally NA, Islam MS. Ibrahim MA, et al. Acta Pharm. 2014 Sep;64(3):311-24. doi: 10.2478/acph-2014-0025. Acta Pharm. 2014. PMID: 25296677 - In Silico Approaches to Identify Polyphenol Compounds as α-Glucosidase and α-Amylase Inhibitors against Type-II Diabetes.
Riyaphan J, Pham DC, Leong MK, Weng CF. Riyaphan J, et al. Biomolecules. 2021 Dec 14;11(12):1877. doi: 10.3390/biom11121877. Biomolecules. 2021. PMID: 34944521 Free PMC article. Review. - Flavonoids as potential agents in the management of type 2 diabetes through the modulation of α-amylase and α-glucosidase activity: a review.
Proença C, Ribeiro D, Freitas M, Fernandes E. Proença C, et al. Crit Rev Food Sci Nutr. 2022;62(12):3137-3207. doi: 10.1080/10408398.2020.1862755. Epub 2021 Jan 11. Crit Rev Food Sci Nutr. 2022. PMID: 33427491 Review.
Cited by
- Phytochemical Screening on Phenolic, Flavonoid Contents, and Antioxidant Activities of Six Indigenous Plants Used in Traditional Thai Medicine.
Tiranakwit T, Puangpun W, Tamprasit K, Wichai N, Siriamornpun S, Srisongkram T, Weerapreeyakul N. Tiranakwit T, et al. Int J Mol Sci. 2023 Aug 30;24(17):13425. doi: 10.3390/ijms241713425. Int J Mol Sci. 2023. PMID: 37686230 Free PMC article. - Glinus lotoides linn. Seed extract as antidiabetic agent: In vitro and in vivo anti-glucolipotoxicity efficacy in Type-II diabetes mellitus.
Sisay W, Andargie Y, Molla M, Tessema G, Singh P. Sisay W, et al. Metabol Open. 2022 May 19;14:100189. doi: 10.1016/j.metop.2022.100189. eCollection 2022 Jun. Metabol Open. 2022. PMID: 35637658 Free PMC article. - Synthesis and bioactivities evaluation of quinazolin-4(3H)-one derivatives as α-glucosidase inhibitors.
Moheb M, Iraji A, Dastyafteh N, Khalili Ghomi M, Noori M, Mojtabavi S, Faramarzi MA, Rasekh F, Larijani B, Zomorodian K, Sadat-Ebrahimi SE, Mahdavi M. Moheb M, et al. BMC Chem. 2022 Nov 15;16(1):97. doi: 10.1186/s13065-022-00885-z. BMC Chem. 2022. PMID: 36380337 Free PMC article. - Diabetes-Related Mechanisms of Action Involved in the Therapeutic Effect of Croton Species: A Systematic Review.
Espinoza-Hernández FA, Moreno-Vargas AD, Andrade-Cetto A. Espinoza-Hernández FA, et al. Plants (Basel). 2023 May 18;12(10):2014. doi: 10.3390/plants12102014. Plants (Basel). 2023. PMID: 37653931 Free PMC article. Review. - Dipterocarpol in Oleoresin of Dipterocarpus alatus Attributed to Cytotoxicity and Apoptosis-Inducing Effect.
Puthongking P, Yongram C, Katekaew S, Sungthong B, Weerapreeyakul N. Puthongking P, et al. Molecules. 2022 May 17;27(10):3187. doi: 10.3390/molecules27103187. Molecules. 2022. PMID: 35630669 Free PMC article.
References
- Goyal R., Jialal I. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2021. Diabetes Mellitus Type 2.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical