How Is Global Warming Affecting Fruit Tree Blooming? "Flowering (Dormancy) Disorder" in Japanese Pear (Pyrus pyrifolia) as a Case Study - PubMed (original) (raw)
Review
How Is Global Warming Affecting Fruit Tree Blooming? "Flowering (Dormancy) Disorder" in Japanese Pear (Pyrus pyrifolia) as a Case Study
Akiyoshi Tominaga et al. Front Plant Sci. 2022.
Abstract
Recent climate change has resulted in warmer temperatures. Warmer temperatures from autumn to spring has negatively affected dormancy progression, cold (de)acclimation, and cold tolerance in various temperate fruit trees. In Japan, a physiological disorder known as flowering disorder, which is an erratic flowering and bud break disorder, has recently emerged as a serious problem in the production of the pome fruit tree, Japanese (Asian) pear (Pyrus pyrifolia Nakai). Due to global warming, the annual temperature in Japan has risen markedly since the 1990s. Surveys of flowering disorder in field-grown and greenhouse-grown Japanese pear trees over several years have indicated that flowering disorder occurs in warmer years and cultivation conditions, and the risk of flowering disorder occurrence is higher at lower latitudes than at higher latitudes. Susceptibility to flowering disorder is linked to changes in the transcript levels of putative dormancy/flowering regulators such as DORMANCY-ASSOCIATED MADS-box (DAM) and FLOWERING LOCUS T (FT). On the basis of published studies, we conclude that autumn-winter warm temperatures cause flowering disorder through affecting cold acclimation, dormancy progression, and floral bud maturation. Additionally, warm conditions also decrease carbohydrate accumulation in shoots, leading to reduced tree vigor. We propose that all these physiological and metabolic changes due to the lack of chilling during the dormancy phase interact to cause flowering disorder in the spring. We also propose that the process of chilling exposure rather than the total amount of chilling may be important for the precise control of dormancy progression and robust blooming, which in turn suggests the necessity of re-evaluation of the characteristics of cultivar-dependent chilling requirement trait. A full understanding of the molecular and metabolic regulatory mechanisms of both dormancy completion (floral bud maturation) and dormancy break (release from the repression of bud break) will help to clarify the physiological basis of dormancy-related physiological disorder and also provide useful strategies to mitigate or overcome it under global warming.
Keywords: DAM; chilling requirement; cold accumulation; dormancy; floral bud maturation; warm temperature.
Copyright © 2022 Tominaga, Ito, Sugiura and Yamane.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
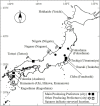
FIGURE 1
Locations of prefectures where Japanese pear is produced. Black markers indicate main production prefectures (>8,000 tons) [statistics from MAFF]1. White markers indicate northernmost and southernmost prefectures producing Japanese pear. Squares indicate locations of flowering disorder surveys.
FIGURE 2
Typical symptoms of flowering disorder at blooming. (A) Healthy (normal) flower. Pear flower bud is a mixed flower bud, with one or two floral primordia (constituted of several florets) and sometimes leafy primordia. (B–D) Flowers with only one or two florets blooming. (E,F) Flowers with shortened peduncle. (G) Flowers with all florets aborted and only elongated leaf primordia. (H) Flower buds located at distal parts (on long or spur shoots) bloomed but those on basal parts were delayed or did not flower.
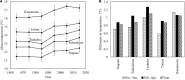
FIGURE 3
Mean temperature and temperature differences over the last 60 years. (A) Changes in decadal mean temperature in main production areas of Japanese pear. (B) Seasonal increase in mean temperature from the 30-year period in 1960–1989 to the subsequent 30-year period (1990–2019). Temperature data were recorded at Japan Meteorological Agency observatories [statistics from JMA]a.
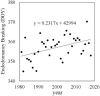
FIGURE 4
Endodormancy breaking date of Japanese pear ‘Kosui’ in Tsukuba. Endodormancy breaking date estimated from the DVR model (Sugiura and Honjo, 1997) and observed hourly temperature. DOY, day of the year (number of days from January 1).
FIGURE 5
Typical appearance of flower buds of two cultivars after removing outer scale leaves. Trees were grown at five localities and observed in the 2015–2016 season. Rectangles indicate floret damage, ovals indicate floret abortion. (A) Healthy florets, (B,C) injured florets, (D,E) (distal) florets aborted in winter (modified from Ito et al., 2018).
FIGURE 6
Proportion of injured florets out of total number of florets counted at blooming (observed in March 2016) (modified from Ito et al., 2018).
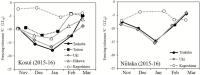
FIGURE 7
Seasonal changes in freezing tolerance of axillary flower buds of two cultivars grown at five or three localities (2015–16 season) (modified from Ito et al., 2018).
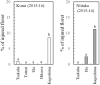
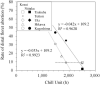
FIGURE 8
Relationship between chill units and rate of distal floret abortion in ‘Kosui’ (open symbols) and ‘Niitaka’ (closed symbols) (modified from Ito et al., 2018).
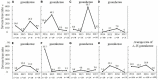
FIGURE 9
Annual trends in severe tree ratez in heated greenhouses (A–H greenhouses) (taken from Tominaga et al., 2019 with the permission by JSHS). zTrees with a flowering rate of lower than 30% were defined as “severe flowering disorder trees.” Proportion of severe trees out of all trees in the greenhouse was defined as the “severe tree rate.”
FIGURE 10
Changes in dormancy- and flowering-related gene expression in normal trees (NTs) and flowering disorder trees (FDTs) (taken from Tominaga et al., 2021 with the permission by JSHS). (A) Transcript levels of PpFT2a. (B) Transcript levels of PpMADS13-3. Relative gene transcript levels were normalized to that of PpHistonH3. Vertical bar indicates standard error (n = 3–4 axillary flower buds). Different letters indicate significant differences at 5% significance level (Tukey–Kramer test).
FIGURE 11
Depth of dormancy in normal trees (NTs) and flowering disorder trees (FDTs) (taken from Tominaga et al., 2021 with the permission by JSHS). (A) Dormancy depth and (B) changes in dormancy depth. Vertical bar indicates standard error (n = 3).
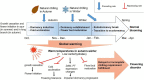
FIGURE 12
Proposed mechanism underlying flowering (dormancy) disorder in Japanese pear due to global warming.
Similar articles
- Comparative phenology of dormant Japanese pear (Pyrus pyrifolia) flower buds: a possible cause of 'flowering disorder'.
Ito A, Sakaue T, Fujimaru O, Iwatani A, Ikeda T, Sakamoto D, Sugiura T, Moriguchi T. Ito A, et al. Tree Physiol. 2018 Jun 1;38(6):825-839. doi: 10.1093/treephys/tpx169. Tree Physiol. 2018. PMID: 29370432 - Physiological differences between bud breaking and flowering after dormancy completion revealed by DAM and FT/TFL1 expression in Japanese pear (Pyrus pyrifolia).
Ito A, Saito T, Sakamoto D, Sugiura T, Bai S, Moriguchi T. Ito A, et al. Tree Physiol. 2016 Jan;36(1):109-20. doi: 10.1093/treephys/tpv115. Epub 2015 Nov 6. Tree Physiol. 2016. PMID: 26546364 - I Want to (Bud) Break Free: The Potential Role of DAM and _SVP_-Like Genes in Regulating Dormancy Cycle in Temperate Fruit Trees.
Falavigna VDS, Guitton B, Costes E, Andrés F. Falavigna VDS, et al. Front Plant Sci. 2019 Jan 10;9:1990. doi: 10.3389/fpls.2018.01990. eCollection 2018. Front Plant Sci. 2019. PMID: 30687377 Free PMC article. Review. - Effects of dormancy progression and low-temperature response on changes in the sorbitol concentration in xylem sap of Japanese pear during winter season.
Ito A, Sugiura T, Sakamoto D, Moriguchi T. Ito A, et al. Tree Physiol. 2013 Apr;33(4):398-408. doi: 10.1093/treephys/tpt021. Epub 2013 Apr 5. Tree Physiol. 2013. PMID: 23564693 - Genetic and molecular regulation of chilling requirements in pear: breeding for climate change resilience.
Gabay G, Flaishman MA. Gabay G, et al. Front Plant Sci. 2024 Apr 26;15:1347527. doi: 10.3389/fpls.2024.1347527. eCollection 2024. Front Plant Sci. 2024. PMID: 38736438 Free PMC article. Review.
Cited by
- Evolutionary and Integrative Analysis of Gibberellin-Dioxygenase Gene Family and Their Expression Profile in Three Rosaceae Genomes (F. vesca, P. mume, and P. avium) Under Phytohormone Stress.
Sabir IA, Manzoor MA, Shah IH, Abbas F, Liu X, Fiaz S, Shah AN, Jiu S, Wang J, Abdullah M, Zhang C. Sabir IA, et al. Front Plant Sci. 2022 Jul 7;13:942969. doi: 10.3389/fpls.2022.942969. eCollection 2022. Front Plant Sci. 2022. PMID: 35874024 Free PMC article. - Integrated transcriptome, metabolome and phytohormone analysis reveals developmental differences between the first and secondary flowering in Castanea mollissima.
Qin C, Du T, Zhang R, Wang Q, Liu Y, Wang T, Cao H, Bai Q, Zhang Y, Su S. Qin C, et al. Front Plant Sci. 2023 Mar 16;14:1145418. doi: 10.3389/fpls.2023.1145418. eCollection 2023. Front Plant Sci. 2023. PMID: 37008486 Free PMC article. - Unlocking Nature's Clock: CRISPR Technology in Flowering Time Engineering.
Hodaei A, Werbrouck SPO. Hodaei A, et al. Plants (Basel). 2023 Nov 29;12(23):4020. doi: 10.3390/plants12234020. Plants (Basel). 2023. PMID: 38068655 Free PMC article. Review. - Genome-Wide Identification of Callose Synthase Family Genes and Their Expression Analysis in Floral Bud Development and Hormonal Responses in Prunus mume.
Zhang M, Cheng W, Wang J, Cheng T, Lin X, Zhang Q, Li C. Zhang M, et al. Plants (Basel). 2023 Dec 14;12(24):4159. doi: 10.3390/plants12244159. Plants (Basel). 2023. PMID: 38140486 Free PMC article. - Possible Mechanism of Sucrose and Trehalose-6-Phosphate in Regulating the Secondary Flower on the Strong Upright Spring Shoots of Blueberry Planted in Greenhouse.
Wu HL, Zhang SL, Feng X, Zhang YQ, Zhou BJ, Cao M, Wang YP, Guo BS, Hou ZX. Wu HL, et al. Plants (Basel). 2024 Aug 23;13(17):2350. doi: 10.3390/plants13172350. Plants (Basel). 2024. PMID: 39273834 Free PMC article.
References
- Bai S., Tuan P. A., Saito T., Ito A., Ubi B. E., Ban Y., et al. (2017). Repression of TERMINAL FLOWER1 primarily mediates floral induction in pear (Pyrus pyrifolia Nakai) concomitant with change in gene expression of plant hormone-related genes and transcription factors. J. Exp. Bot. 68 4899–4914. 10.1093/jxb/erx296 - DOI - PMC - PubMed
- Bielenberg D. G., Wang Y. E., Li Z., Zhebentyayeva T., Fan S., Reighard G. L., et al. (2008). Sequencing and annotation of the evergrowing locus in peach [Prunus persica (L.) Batsch] reveals a cluster of six MADS-box transcription factors as candidate genes for regulation of terminal bud formation. Tree Gen. Genome 4 495–507. 10.1007/s11295-007-0126-9 - DOI
Publication types
LinkOut - more resources
Full Text Sources