Cingulo-Opercular and Frontoparietal Network Control of Effort and Fatigue in Mild Traumatic Brain Injury - PubMed (original) (raw)
Cingulo-Opercular and Frontoparietal Network Control of Effort and Fatigue in Mild Traumatic Brain Injury
Amy E Ramage et al. Front Hum Neurosci. 2022.
Abstract
Neural substrates of fatigue in traumatic brain injury (TBI) are not well understood despite the considerable burden of fatigue on return to productivity. Fatigue is associated with diminishing performance under conditions of high cognitive demand, sense of effort, or need for motivation, all of which are associated with cognitive control brain network integrity. We hypothesize that the pathophysiology of TBI results in damage to diffuse cognitive control networks, disrupting coordination of moment-to-moment monitoring, prediction, and regulation of behavior. We investigate the cingulo-opercular (CO) and frontoparietal (FP) networks, which are engaged to sustain attention for task and maintain performance. A total of 61 individuals with mild TBI and 42 orthopedic control subjects participated in functional MRI during performance of a constant effort task requiring altering the amount of effort (25, 50, or 75% of maximum effort) utilized to manually squeeze a pneumostatic bulb across six 30-s trials. Network-based statistics assessed within-network organization and fluctuation with task manipulations by group. Results demonstrate small group differences in network organization, but considerable group differences in the evolution of task-related modulation of connectivity. The mild TBI group demonstrated elevated CO connectivity throughout the task with little variation in effort level or time on task (TOT), while CO connectivity diminished over time in controls. Several interregional CO connections were predictive of fatigue in the TBI group. In contrast, FP connectivity fluctuated with task manipulations and predicted fatigue in the controls, but connectivity fluctuations were delayed in the mild traumatic brain injury (mTBI) group and did not relate to fatigue. Thus, the mTBI group's hyper-connectivity of the CO irrespective of task demands, along with hypo-connectivity and delayed peak connectivity of the FP, may allow for attainment of task goals, but also contributes to fatigue. Findings are discussed in relation to performance monitoring of prediction error that relies on internal cues from sensorimotor feedback during task performance. Delay or inability to detect and respond to prediction errors in TBI, particularly evident in bilateral insula-temporal CO connectivity, corresponds to day-to-day fatigue and fatigue during task performance.
Keywords: brain injury – traumatic brain injury; cognitive control networks; effort; fatigue; neuroimaging.
Copyright © 2022 Ramage, Ray, Franz, Tate, Lewis and Robin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
FIGURE 1
The mild traumatic brain injury (mTBI) and Orthopedic Control groups did not differ significantly in time constant (TC), but the Control group showed consistent decline, or evidenced fatigue, as effort level increased. Error bars represent 95% confidence intervals.
FIGURE 2
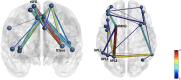
Group differences in (A) cingulo-opercular and (B) frontoparietal networks demonstrate stronger connectivity in the control group than in the mTBI group. The edge with the largest group difference in the CO network was the right superior frontal gyrus–left claustrum [t(103) = 3.76, p < 0.001, red]. Edges with the largest group differences in the FP network were the left inferior parietal lobule 1–left inferior parietal lobule 3 [t(103) = 3.73, p < 0.001], and left middle frontal gyrus 1–left inferior parietal lobule 2 [t(103) = 3.96, p < 0.001].
FIGURE 3
Positive main effects of effort level and time on task (TOT) for all the participants combined were robust, but the FP network was more diffusely engaged in effort level task manipulation. Edge thickness = _t_-value for the edge in the effect.
FIGURE 4
Both groups demonstrated robust main effects for effort level and TOT with similar spatial distributions across effects (effort or TOT) and across groups (A). However, the effects were stronger in the control group (_t_-values presented by the color spectrum and edge thickness). For both groups, the edge with strongest effects (for effort and TOT) was the left medial frontal gyrus–right medial frontal gyrus [mTBI effort effect: t(63) = 5.24, control effort effect: t(40) = 11.81; mTBI TOT effect: t(63) = 4.61, control TOT effect: t(40) = 11.4, all _p_s < 0.001]. Connectivity between the left insular and medial frontal nodes was stronger for the effort effect in both groups, but only one edge connecting these brain regions was significant in the mTBI group (left precentral gyrus-left claustrum). Connections between medial frontal and insular or temporal nodes were absent in TOT for the mTBI group. The (B) heatmaps and (C) pairplots present correlation strengths and distributions (C, center diagonal) for the edges that were significant for effort level and TOT effects by group. The heatmaps show that for the controls (B, bottom), connectivity was strongest at the beginning of the task (25% effort level), indicated by darker red only in the top left corner, while connectivity was strong throughout the task in the mTBI group (B, top). The pairplots demonstrate that the FC over the course of the task was similar by group but more kurtotic in the OC group early in the task (at 25% second and 50% first). FC was more kurtotic at the end of the task for the mTBI group. The heatmaps and pairplots also present the negative relationship between edge length and FC in both groups, which was strongest at the 50 and 75% effort levels in the mTBI group, but more so for the 75% effort level in the OC group. The latter may indicate a “preference” for shorter Euclidean distances when a task becomes more effortful. (D) Node degree was presented as the number of connections each node was engaged in, standardized for the total number of significant edges in each effect (edge ratio), with more nodes engaged for the effect of effort level in the controls, but more nodes engaged in the TOT effect for the mTBI group. Error bars, standard error. lmedFG, left medial frontal gyrus; rIPL, right inferior parietal lobule; rMFG, right middle frontal gyrus; lSFG, left superior frontal gyrus; lCing, left mid cingulate cortex; rINS1, right insula 1; rSFG, right superior frontal gyrus; rmedFG, right medial frontal gyrus; lPcnt, left precentral gyrus; rSTG, right superior temporal gyrus; lClaus, left claustrum; lSTG, left superior temporal gyrus; lACC, left anterior cingulate cortex; and raINS, right anterior insula.
FIGURE 5
Frontoparietal network connectivity by group. Both groups demonstrated robust main effects for effort level and TOT with similar spatial distributions across effects (effort or TOT) and across groups. The effects were stronger in the control group (_t_-values presented by the color spectrum) than in the mTBI group but with small effect size (d = 0.25). Spatial distribution was larger for the effort level than for TOT in both groups, but effect sizes did not differ (mTBI d = 0.31 for both, control d = 0.41 for effort, d = 0.4 for TOT). However, on visual inspection of the network, the distribution of significant edges and stronger edges (_t_-values presented by color spectrum and edge thickness), was greater among the frontal nodes than the parietal nodes in the mTBI group. In contrast, connectivity was well distributed across nodes in the control group. This pattern, i.e., more and stronger connections in frontal than parietal nodes, is also evident in the TOT effect. The (B) heatmaps and (C) pairplot present the correlation strengths and distributions (C, center diagonal) for edges that were significant at the effort level and TOT effects by group, and the scatter plots present the relationships between each of the effort levels by TOT. The heatmaps demonstrate that the mTBI group (B, top) had stronger FC throughout the task with only slight increases in FC for the first half of each trial. In contrast, the connectivity of the controls varied consistently for the first half of the 50 and 75% effort levels. The pairplot presents the distributions of FP connectivity strength for the significant edges by group, and while the NBS results did not indicate strong group differences in the FP (evident in A), these plots make evident the differing patterns of FC relationships by group. First, the distributions differ in the OC by TOT, with a more kurtotic peak for the first trial at 25% that flattens in the second half at 25%, and then remain near the same for the other effort levels with slight skew to the right (stronger connectivity). This was not the case in the mTBI group where the distribution was kurtotic for all effort levels. In both groups, there were bimodal distributions, with smaller numbers of participant data skewed to the left (weaker connectivity). Also evident in the (B) heatmaps is the negative correlation of edge length with FC. This negative correlation was slightly weaker in the mTBI group, and there was a trend toward more negative FC path length correlations with increasing effort level in the OC group. (D) Node degree, presented as the number of nodes by the total number of edges for each effect and group, demonstrated group differences in the numbers of connections each node was engaged in, with higher degree in frontal nodes for the mTBI group relative to the control group. The involvement of frontal nodes was particularly higher for the mTBI group in the TOT effect. lPcnt, left precentral gyrus; rtriIFG, right inferior frontal gyrus triangularis 1; ltriIFG, left inferior frontal gyrus triangularis 1; lIPL, left inferior parietal lobule 1; lMFG, left middle frontal gyrus 1; rITG, right inferior temporal gyrus 1; rorbSFG, right superior frontal gyrus orbitalis 1; rorbMFG, right middle frontal gyrus orbitalis 1; rPcnt, right precentral gyrus 1; lPcnt2, left precentral gyrus 2; lMFG2, left middle frontal gyrus 2; rMFG, right middle frontal gyrus 1; rIPL, right inferior parietal lobule 1; lIPL2, left inferior parietal lobule 2; rIPL2, right inferior parietal lobule 2; rMFG2, right middle frontal gyrus 2; rAG, right angular gyrus 1; lIPL3, left inferior parietal lobule 3; rMFG3, right middle frontal gyrus 3; lMFG2, left middle frontal gyrus 2; lorbMFG, left middle frontal gyrus orbitalis 1; rAG2, right angular gyrus 2; rorbMFG2, right middle frontal gyrus orbitalis 2; ltriIFG2, left inferior frontal gyrus triangularis 2; lsupmedFG, left superior medial frontal gyrus.
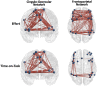
FIGURE 6
Significant predictors (edge thickness = b coefficient size) of trait (FSS) or state (TC) fatigue tended to involve edges of the CO network in the mTBI group (top row), but not the FP network in the control group (bottom row). The connectivity of several CO edges was predictive of trait fatigue (FSS score) in the mTBI group, specifically weaker connectivity of two edges in the latter part of the 50% effort level second half or 75% effort level first half, and stronger the connectivity of three edges during the most effortful (50 and 75% effort levels) in the second half of the trials (longer TOT). In addition, two FP edges in the highest effort level (75%) at longer TOT (second half) were predictive of trait fatigue in the mTBI group. In contrast, it was largely FP edge connectivity, and specifically the parietal edges, along with only two edges of the CO, that were predictive of trait fatigue in the OC group. Similar group differences were seen for prediction of state fatigue (time constant during the constant effort task), with several FP edges predicting fatigue in the OC group. Interestingly, the weaker connectivity between the right superior temporal gyrus (Mortera et al., 2018) and the left claustrum (Bigler, 2017) in the mTBI group predicted more trait and state fatigue. Note: Edge thickness indicates predictive strength (absolute beta value) from the generalized linear model predicting FSS score (reported in Table 4) or the generalized mixed model predicting TC (reported in Table 5). Cingulo-opercular network: 1, left medial frontal gyrus; 2, right inferior parietal lobule; 3, right middle frontal gyrus; 4, left superior frontal gyrus; 5, left mid cingulate cortex; 6, right insula 1; 7, right superior frontal gyrus; 8, right medial frontal gyrus; 9, left precentral gyrus; 10, right superior temporal gyrus; 11, left claustrum; 12, left superior temporal gyrus; 13, left anterior cingulate cortex; and 14, right anterior insula. Frontoparietal network: 1, left precentral gyrus; 2, right inferior frontal gyrus triangularis 1; 3, left inferior frontal gyrus triangularis 1; 4, left inferior parietal lobule 1; 5, left middle frontal gyrus 1; 6, right inferior temporal gyrus 1; 7, right superior frontal gyrus orbitalis 1; 8, right middle frontal gyrus orbitalis 1; 9, right precentral gyrus 1; 10, left precentral gyrus 2; 11, left middle frontal gyrus 2; 12, right middle frontal gyrus 1; 13, right inferior parietal lobule 1; 14, left inferior parietal lobule 2; 15, right inferior parietal lobule 2; 16, right middle frontal gyrus 2; 17, right angular gyrus 1; 18, left inferior parietal lobule 3; 19, right middle frontal gyrus 3; 20, left middle frontal gyrus 3; 21, left middle frontal gyrus orbitalis 1; 22, right angular gyrus 2; 23, right middle frontal gyrus orbitalis 2; 24, left inferior frontal gyrus triangularis 2; and 25, left superior medial frontal gyrus.
References
- Ashburner J., Friston K. J. (2005). Unified segmentation. NeuroImage 26 839–851. - PubMed
LinkOut - more resources
Full Text Sources