Deficient Autophagy in Microglia Aggravates Repeated Social Defeat Stress-Induced Social Avoidance - PubMed (original) (raw)
. 2022 Feb 16:2022:7503553.
doi: 10.1155/2022/7503553. eCollection 2022.
Zhiqian Yu 1, Ryo Hirayama 1, Masa Nakasato 1, Yoshie Kikuchi 1, Chiaki Ono 1, Hiroshi Komatsu 1, Miharu Nakanishi 2, Hatsumi Yoshii 2, David Stellwagen 3, Tomoyuki Furuyashiki 4, Masaaki Komatsu 5, Hiroaki Tomita 1 6
Affiliations
- PMID: 35222638
- PMCID: PMC8866015
- DOI: 10.1155/2022/7503553
Deficient Autophagy in Microglia Aggravates Repeated Social Defeat Stress-Induced Social Avoidance
Mai Sakai et al. Neural Plast. 2022.
Abstract
Major depressive disorder (MDD) is associated with repeated exposure to environmental stress. Autophagy is activated under various stress conditions that are associated with several diseases in the brain. This study was aimed at elucidating the autophagy signaling changes in the prefrontal cortex (PFC) under repeated social defeat (RSD) to investigate the involvement of microglial autophagy in RSD-induced behavioral changes. We found that RSD stress, an animal model of MDD, significantly induced initial autophagic signals followed by increased transcription of autophagy-related genes (Atg6, Atg7, and Atg12) in the PFC. Similarly, significantly increased transcripts of ATGs (Atg6, Atg7, Atg12, and Atg5) were confirmed in the postmortem PFC of patients with MDD. The protein levels of the prefrontal cortical LC3B were significantly increased, whereas p62 was significantly decreased in the resilient but not in susceptible mice and patients with MDD. This indicates that enhanced autophagic flux may alleviate stress-induced depression. Furthermore, we identified that FKBP5, an early-stage autophagy regulator, was significantly increased in the PFC of resilient mice at the transcript and protein levels. In addition, the resilient mice exhibited enhanced autophagic flux in the prefrontal cortical microglia, and the autophagic deficiency in microglia aggravated RSD-induced social avoidance, indicating that microglial autophagy involves stress-induced behavioral changes.
Copyright © 2022 Mai Sakai et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Figure 1
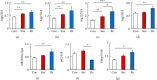
The effects of chronic restraint stress on behavior. (a) Schedule of behavioral experiments. Beginning at day 0 and concluding on day 10, each animal was assigned to the RSD group. From day 11 to day 15, the mice were subjected to SIT, EPM, and SPT each day, and brain samples were collected at day 16. (b) Definitions of the social interaction zone and the social avoidance zone (grey and black rectangles, respectively). (c) Horizontal scatterplot depicting the distribution of interaction ratios for control. The durations in the social interaction zone (d) and social avoidance zone (e) in wild-type (WT) mice with or without RSD. (f) The duration of the open arms in the elevated plus maze test as an index for anxiety of wild-type mice. (g) The proportion of the sucrose intake as an index for depression of wild-type mice after RSD. RSD: repeated social defeat; SIT: social interaction test; Con: control mice; Sus: susceptible mice; Re: resilient mice; SI: social interaction; SA: social avoidance. One-way ANOVA followed by Turkey's post hoc test was applied to all comparisons (Con, n = 12; Sus, n = 16; Res, n = 5). Data are presented as the mean ± SEM.
Figure 2
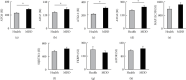
Repeated social defeat stress induced the expression of autophagic signaling in the prefrontal cortex. (a) Levels of the mRNA encoding the autophagic signaling marker Atg6 relative to those of S18. (b) Levels of the mRNA encoding the autophagic signaling marker Atg7 relative to those of S18. (c) Levels of the mRNA encoding the autophagic signaling marker Atg12 relative to those of S18. (d) Levels of the mRNA encoding the autophagic signaling marker Atg5 relative to those of S18. (e) Levels of the mRNA encoding the autophagic signaling marker Map1lc3b relative to those of S18. (f) Levels of the mRNA encoding the autophagic signaling marker p62 relative to those of S18. (g) Levels of the mRNA encoding the autophagic activator Fkbp5 relative to those of S18. Con: control mice; Sus: susceptible mice; Re: resilient mice. One-way ANOVA followed by Turkey's post hoc test was applied to all comparisons (Con, n = 12; Sus, n = 16; Res, n = 5). Data are presented as the mean ± SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 vs. Con or Re.
Figure 3
Autophagic signaling changes in the postmortem prefrontal cortex of patients with depression. (a) The expression of ATG6 mRNA in the postmortem tissue. (b) The expression of ATG7 mRNA in the postmortem tissue. (c) The expression of ATG12 mRNA in the postmortem tissue. (d) The expression of ATG5 mRNA in the postmortem tissue. (e) The expression of MAP1LC3B mRNA in the postmortem tissue. (f) The expression of SQRTM1 mRNA in the postmortem tissue. (g) The expression of FKBP5 mRNA in the postmortem tissue. (h) The expression of mTOR mRNA in the postmortem tissue. SI: signal intensity; Health: healthy control; MDD: major depressive disorder. Health, n = 56; MDD, n = 29. The random variance _t_-test with the Benjamini–Hochberg false discovery (FDR) correction was applied. Data are presented as the mean ± SEM. ∗FDR q value < 0.05.
Figure 4
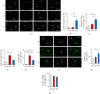
Repeated social defeat stress enhanced autophagy activation in the prefrontal cortex of resilient mice. (a–e) Representative images (a) and quantitative analyses (b–e) of immunostaining for LC3B-puncta colocalized with p62 signals in the PFC of CX3CR1GFP/+ mice without RSD (Con) and susceptible and resilient mice 24 h after the last session of RSD. In the merged images in (a), CX3CR1, LC3B, and p62 are shown in green, white, and red, respectively. Scale bars, 10 μ_m. (b) was determined by calculating the density of the LC3B area/total area. (c) was determined by calculating the density of the positive LC3B signals in each microglia. (e) was determined by calculating the density of the p62 area/total area. (f) was determined by calculating the density of the positive p62 signals in each microglia. (f–h) Representative images (f) and quantitative analyses (g, h) of immunostaining for FKBP5 in the PFC of Cx3CR1GFP/+ mice without RSD (Con) and susceptible and resilient mice 24 h after the last session of RSD. In the merged images in (f), CX3CR1 and FKBP5 are shown in green and red, respectively. Scale bars, 10 μ_m. (g) was determined by calculating the density of the FKBP5 area/total area. (h) was determined by calculating the density of the positive FKBP5 signals in microglia. Con: control mice; Sus: susceptible mice; Re: resilient mice. One-way ANOVA followed by Turkey's post hoc test was applied to all comparisons. Data are presented as the mean ± SEM. Con, n = 4; Sus, n = 4; Res, n = 4. ∗_P < 0.05, ∗∗_P < 0.01, and ∗∗P < 0.001 vs. Con or Re.
Figure 5
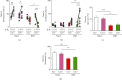
The effect of microglial autophagy deficient on behavior changes. (a, b) The levels of social interaction (a) and social avoidance (b) in Cre-negative mice and Cre+ mice with or without RSD. The duration in the interaction (a) or avoidance (b) zone without and with an ICR mouse was analyzed and is shown (n = 7). One-way ANOVA followed by Turkey's post hoc test was applied to all comparisons. Data are presented as the mean ± SEM. P < 0.01 vs. ICR (-) Cre-negative. #P < 0.05 vs. ICR (-) Cre+. ∗P < 0.05, ∗∗∗P < 0.001 vs. ICR (+) Cre-negative. (c) The proportions of the time for the open arms in the elevated plus maze test as an index for anxiety of Cre-negative and Cre+ mice (n = 7). (d) The proportions of the sucrose intake in the sucrose preference test as an index for depression of Cre-negative and Cre+ mice (n = 7). One-way ANOVA followed by Turkey's post hoc test was applied to all comparisons. Data are presented as the mean ± SEM. ∗P < 0.05, ∗∗∗P < 0.001 vs. Cre-negative or Cre+; repeated social defeat, Atg+/+; Cre-negative mice, Atg-/-; and Cre+;Atgflox/flox mice. Naive: nonstressed; RSD: repeated social defeat.
Similar articles
- HMGB1/STAT3/p65 axis drives microglial activation and autophagy exert a crucial role in chronic Stress-Induced major depressive disorder.
Xu K, Wang M, Wang H, Zhao S, Tu D, Gong X, Li W, Liu X, Zhong L, Chen J, Xie P. Xu K, et al. J Adv Res. 2024 May;59:79-96. doi: 10.1016/j.jare.2023.06.003. Epub 2023 Jun 13. J Adv Res. 2024. PMID: 37321346 Free PMC article. - Ketogenic diet changes microglial morphology and the hippocampal lipidomic profile differently in stress susceptible versus resistant male mice upon repeated social defeat.
González Ibáñez F, Halvorson T, Sharma K, McKee CG, Carrier M, Picard K, Vernoux N, Bisht K, Deslauriers J, Lalowski M, Tremblay MÈ. González Ibáñez F, et al. Brain Behav Immun. 2023 Nov;114:383-406. doi: 10.1016/j.bbi.2023.09.006. Epub 2023 Sep 7. Brain Behav Immun. 2023. PMID: 37689276 - Enhanced fear memory after social defeat in mice is dependent on interleukin-1 receptor signaling in glutamatergic neurons.
Goodman EJ, Biltz RG, Packer JM, DiSabato DJ, Swanson SP, Oliver B, Quan N, Sheridan JF, Godbout JP. Goodman EJ, et al. Mol Psychiatry. 2024 Aug;29(8):2321-2334. doi: 10.1038/s41380-024-02456-1. Epub 2024 Mar 8. Mol Psychiatry. 2024. PMID: 38459193 Free PMC article. - The neuroimmunology of social-stress-induced sensitization.
Biltz RG, Sawicki CM, Sheridan JF, Godbout JP. Biltz RG, et al. Nat Immunol. 2022 Nov;23(11):1527-1535. doi: 10.1038/s41590-022-01321-z. Epub 2022 Nov 11. Nat Immunol. 2022. PMID: 36369271 Free PMC article. Review. - Awakening the dormant: Role of axonal guidance cues in stress-induced reorganization of the adult prefrontal cortex leading to depression-like behavior.
Mahmud A, Avramescu RG, Niu Z, Flores C. Mahmud A, et al. Front Neural Circuits. 2023 Mar 24;17:1113023. doi: 10.3389/fncir.2023.1113023. eCollection 2023. Front Neural Circuits. 2023. PMID: 37035502 Free PMC article. Review.
Cited by
- The Metabolic Basis for Nervous System Dysfunction in Alzheimer's Disease, Parkinson's Disease, and Huntington's Disease.
Maiese K. Maiese K. Curr Neurovasc Res. 2023;20(3):314-333. doi: 10.2174/1567202620666230721122957. Curr Neurovasc Res. 2023. PMID: 37488757 Free PMC article. - The role of microglial autophagy in Parkinson's disease.
Zhu R, Luo Y, Li S, Wang Z. Zhu R, et al. Front Aging Neurosci. 2022 Nov 1;14:1039780. doi: 10.3389/fnagi.2022.1039780. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36389074 Free PMC article. Review. - Cellular Metabolism: A Fundamental Component of Degeneration in the Nervous System.
Maiese K. Maiese K. Biomolecules. 2023 May 11;13(5):816. doi: 10.3390/biom13050816. Biomolecules. 2023. PMID: 37238686 Free PMC article. Review. - Autophagy-Unlocking New Dimensions in the Pathology and Treatment of Depression.
Luo Q, Zhao Y, Ren P, Liu X, Chen Y, Ying Q, Zhou J. Luo Q, et al. Cells. 2025 May 28;14(11):795. doi: 10.3390/cells14110795. Cells. 2025. PMID: 40497971 Free PMC article. Review. - Glial Markers of Suicidal Behavior in the Human Brain-A Systematic Review of Postmortem Studies.
Yamamoto M, Sakai M, Yu Z, Nakanishi M, Yoshii H. Yamamoto M, et al. Int J Mol Sci. 2024 May 25;25(11):5750. doi: 10.3390/ijms25115750. Int J Mol Sci. 2024. PMID: 38891940 Free PMC article. Review.
References
- Sartorius N. The economic and social burden of depression. Journal of Clinical Psychiatry . 2001;62(Suppl 15):8–11. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous