Aragonite dissolution protects calcite at the seafloor - PubMed (original) (raw)
Aragonite dissolution protects calcite at the seafloor
Olivier Sulpis et al. Nat Commun. 2022.
Abstract
In the open ocean, calcium carbonates are mainly found in two mineral forms. Calcite, the least soluble, is widespread at the seafloor, while aragonite, the more soluble, is rarely preserved in marine sediments. Despite its greater solubility, research has shown that aragonite, whose contribution to global pelagic calcification could be at par with that of calcite, is able to reach the deep-ocean. If large quantities of aragonite settle and dissolve at the seafloor, this represents a large source of alkalinity that buffers the deep ocean and favours the preservation of less soluble calcite, acting as a deep-sea, carbonate version of galvanization. Here, we investigate the role of aragonite dissolution on the early diagenesis of calcite-rich sediments using a novel 3D, micrometric-scale reactive-transport model combined with 3D, X-ray tomography structures of natural aragonite and calcite shells. Results highlight the important role of diffusive transport in benthic calcium carbonate dissolution, in agreement with recent work. We show that, locally, aragonite fluxes to the seafloor could be sufficient to suppress calcite dissolution in the top layer of the seabed, possibly causing calcite recrystallization. As aragonite producers are particularly vulnerable to ocean acidification, the proposed galvanizing effect of aragonite could be weakened in the future, and calcite dissolution at the sediment-water interface will have to cover a greater share of CO2 neutralization.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Fig. 1. Dissolution of natural marine CaCO3 grains after a minute in suspension in water.
The top row shows the water saturation state of calcite (a–c) and aragonite (d) while on the bottom row the corresponding calcite (e–g) and aragonite (h) dissolution rates are displayed.
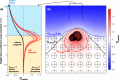
Fig. 2. Effects of the dissolution of a pteropod shell on the saturation state with respect to calcite across the sediment–water interface.
a Depth profile of the saturation state with respect to calcite. The blue circle represents the bottom-water value. The black depth profile stands for a case without aragonite, the red depth profile represents the situation with aragonite shown on the (b) panel. Each depth profile is computed as the mean amongst all data points within the central 850 μm × 850 μm column, which corresponds to the size of the pteropod shell, plus and minus one standard deviation. The extent of the colored envelope surrounding the mean profiles stands for the standard deviation. b Depth transect of water saturation state with respect to calcite, with contours for three selected saturation state values.
Fig. 3. Dissolution of calcite grains in a sediment bed capped with a dissolving aragonite pteropod.
The pteropod is shown in a white mesh. Color gradients indicate surface calcite dissolution rates. The white lining represents a saturation state with respect to calcite of unity, i.e., a transition from undersaturation to supersaturation with respect to calcite that is caused by the dissolving aragonite pteropod.
Fig. 4. Likely main loci of aragonite-based calcite galvanization.
a Map showing in blue the surface area of seafloor that is located below the current calcite saturation depth and the preindustrial calcite compensation depth, i.e., the calcite lysocline; both fields are from ref. . The four yellow stars in the North Pacific correspond to the three sites where ref. observed aragonite in the water column below the aragonite saturation depth and the site where ref. measured pteropod genetic material in bottom waters. b Zonal integral of the seafloor surface area within the calcite lysocline. c Zonal integrals of the surface productions of aragonite (in red) and calcite (in black) in the uppermost 100 m of the surface ocean in ESM2M (solid) and ESM2G (dashed), averaged over 100 yr after a 1000 yr spinup; taken from ref. .
Similar articles
- Current CaCO3 dissolution at the seafloor caused by anthropogenic CO2.
Sulpis O, Boudreau BP, Mucci A, Jenkins C, Trossman DS, Arbic BK, Key RM. Sulpis O, et al. Proc Natl Acad Sci U S A. 2018 Nov 13;115(46):11700-11705. doi: 10.1073/pnas.1804250115. Epub 2018 Oct 29. Proc Natl Acad Sci U S A. 2018. PMID: 30373837 Free PMC article. - Effects of lower surface ocean pH upon the stability of shallow water carbonate sediments.
Tynan S, Opdyke BN. Tynan S, et al. Sci Total Environ. 2011 Feb 15;409(6):1082-6. doi: 10.1016/j.scitotenv.2010.12.007. Epub 2011 Jan 5. Sci Total Environ. 2011. PMID: 21211824 - Seawater Mg/Ca controls polymorph mineralogy of microbial CaCO3: a potential proxy for calcite-aragonite seas in Precambrian time.
Ries JB, Anderson MA, Hill RT. Ries JB, et al. Geobiology. 2008 Mar;6(2):106-19. doi: 10.1111/j.1472-4669.2007.00134.x. Geobiology. 2008. PMID: 18380873 - The Dissolution Rate of CaCO3 in the Ocean.
Adkins JF, Naviaux JD, Subhas AV, Dong S, Berelson WM. Adkins JF, et al. Ann Rev Mar Sci. 2021 Jan;13:57-80. doi: 10.1146/annurev-marine-041720-092514. Epub 2020 Sep 18. Ann Rev Mar Sci. 2021. PMID: 32946363 Review. - Monohydrocalcite: a promising remediation material for hazardous anions.
Fukushi K, Munemoto T, Sakai M, Yagi S. Fukushi K, et al. Sci Technol Adv Mater. 2011 Oct 10;12(6):064702. doi: 10.1088/1468-6996/12/6/064702. eCollection 2011 Dec. Sci Technol Adv Mater. 2011. PMID: 27877452 Free PMC article. Review.
Cited by
- The elements of life: A biocentric tour of the periodic table.
Remick KA, Helmann JD. Remick KA, et al. Adv Microb Physiol. 2023;82:1-127. doi: 10.1016/bs.ampbs.2022.11.001. Epub 2023 Jan 30. Adv Microb Physiol. 2023. PMID: 36948652 Free PMC article. - Enhanced clay formation key in sustaining the Middle Eocene Climatic Optimum.
Krause AJ, Sluijs A, van der Ploeg R, Lenton TM, Pogge von Strandmann PAE. Krause AJ, et al. Nat Geosci. 2023;16(8):730-738. doi: 10.1038/s41561-023-01234-y. Epub 2023 Jul 31. Nat Geosci. 2023. PMID: 37564379 Free PMC article. - Calcium Carbonate Dissolution from the Laboratory to the Ocean: Kinetics and Mechanism.
Batchelor-McAuley C, Yang M, Rickaby REM, Compton RG. Batchelor-McAuley C, et al. Chemistry. 2022 Dec 6;28(68):e202202290. doi: 10.1002/chem.202202290. Epub 2022 Oct 1. Chemistry. 2022. PMID: 36037025 Free PMC article. Review.
References
- Hayes, C. T. et al. Global ocean sediment composition and burial flux in the deep sea. Glob. Biogeochem. Cycles35, e2020GB006769 (2021). - PubMed
- Archer DE. An atlas of the distribution of calcium carbonate in sediments of the deep sea. Glob. Biogeochem. Cycles. 1996;10:159–174.
- Archer D, et al. Atmospheric lifetime of fossil fuel carbon dioxide. Annu. Rev. Earth Planet. Sci. 2009;37:117–134.
- Milliman JD. Production and accumulation of calcium carbonate in the ocean: budget of a nonsteady state. Glob. Biogeochem. Cycles. 1993;7:927–957.
- Smith SV, Mackenzie FT. The role of CaCO3 reactions in the contemporary oceanic CO2 cycle. Aquat. Geochem. 2016;22:153–175.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials