Adolescence is a sensitive period for prefrontal microglia to act on cognitive development - PubMed (original) (raw)
. 2022 Mar 4;8(9):eabi6672.
doi: 10.1126/sciadv.abi6672. Epub 2022 Mar 2.
Anina S von Arx 1, Natalia Cruz-Ochoa 2, Kara Dawson 1, Andranik Ivanov 3, Flavia S Mueller 1, Han-Yu Lin 1, René Amport 1, Wiebke Mildenberger 4, Daniele Mattei 1 5, Dieter Beule 3 6, Csaba Földy 2 7, Melanie Greter 4, Tina Notter 1 8, Urs Meyer 1 7
Affiliations
- PMID: 35235358
- PMCID: PMC8890703
- DOI: 10.1126/sciadv.abi6672
Adolescence is a sensitive period for prefrontal microglia to act on cognitive development
Sina M Schalbetter et al. Sci Adv. 2022.
Abstract
The prefrontal cortex (PFC) is a cortical brain region that regulates various cognitive functions. One distinctive feature of the PFC is its protracted adolescent maturation, which is necessary for acquiring mature cognitive abilities in adulthood. Here, we show that microglia, the brain's resident immune cells, contribute to this maturational process. We find that transient and cell-specific deficiency of prefrontal microglia in adolescence is sufficient to induce an adult emergence of PFC-associated impairments in cognitive functions, dendritic complexity, and synaptic structures. While prefrontal microglia deficiency in adolescence also altered the excitatory-inhibitory balance in adult prefrontal circuits, there were no cognitive sequelae when prefrontal microglia were depleted in adulthood. Thus, our findings identify adolescence as a sensitive period for prefrontal microglia to act on cognitive development.
Figures
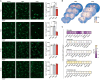
Fig. 1.. Effectiveness, transiency, and selectivity of CDS-induced microglia depletion in the PFC of adolescent mice.
(A) Representative images of Iba1+ microglia (green) in the medial PFC of adolescent mice receiving sham surgery or bilateral intra-PFC injection of PBS or CDS. The images were taken 1, 5, 10, and 20 days post-injection (dpi). The bar graphs show the number of Iba1+ microglia in the medial PFC at different dpi intervals. *P < 0.05, **P < 0.01, and ***P < 0.001 [post hoc tests following ANOVA at 1 dpi: F(2,12) = 12.7, P < 0.01; at 5 dpi: F(2,12) = 140.5, P < 0.001]; N = 5 mice per group and dpi. (B) Color-coded overlays of coronal PFC sections taken from five mice per group (PBS or CDS), wherein each dot represents an Iba1+ microglial cell. Note the reduction of microglial cell density in the medial portion of PFC of CDS mice, as indicated by the symbol (*). (C) Expression of genes [log2 fold change (FC)] defining microglia, astrocytes, neurons, oligodendrocytes, and endothelial cells in CDS relative to PBS mice at 5 dpi; significantly altered genes are denoted with the symbol (*), based on FDR q < 0.05, as provided in fig. S6 and table S1; N = 5 mice per group.
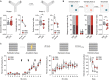
Fig. 2.. Prefrontal microglia deficiency in adolescence disrupts adult cognitive functions.
(A) Phase 1 (D, dummy object; M, unfamiliar mouse) and phase 2 (F, familiar mouse; N, novel mouse) of the social interaction test. The bar plots show the social preference index (phase 1) and social memory index (phase 2), whereas the line plots depict absolute exploration times in either phase. §P < 0.001 [overall main effect of object: F(1,18) = 20.7]; *P < 0.05 [t(18) = 2.84]; +P < 0.01 [main effect of object in PBS mice: F(1,9) = 16.7]. (B) The percentage plots depict the percentage amount of time exploring the left (L) or right (R) object in sample phases 1 and 2 of the temporal order memory test. The bar plot shows the temporal order memory index during the test phase. **P < 0.01 [t(18) = 2.98]. (C) Percent time freezing during the habituation, acquisition, and expression phases (left and middle line plots) and extinction rate (% change from freezing levels measured during the expression phase; right line plot) during the contextual fear test. The arrows indicate the presentation of foot shock. **P < 0.01, based on post hoc tests following ANOVA [treatment × days interaction: F(3,54) = 2.9, P < 0.05]. N = 10 mice in each group and test.
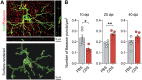
Fig. 3.. Prefrontal microglia deficiency in adolescence leads to dynamic changes in microglial uptake of synaptic particles.
(A) The photomicrograph shows a representative Z-stack image of a double-IF stain using Iba1 (green) as microglial marker and Bassoon (red) as presynaptic marker before (top) and after (bottom) surface rendering and reconstruction with Imaris image analysis software. Bassoon+ presynaptic puncta colocalizing with Iba1+ microglia appear in yellow in the unprocessed IF stain, whereas Bassoon+ presynaptic puncta residing within microglia appear as red dots in the reconstructed image. (B) Quantification of Bassoon+ presynaptic puncta residing within microglia at different days after injection. Note that the number of Bassoon+ synaptic puncta was decreased in prefrontal microglia of CDS-injected mice at 10 dpi [*P < 0.05, t(8) = 2.72], whereas they were increased in CDS mice at 20 dpi [**P < 0.01, t(8) = 4.28]. There were no significant group differences at 40 dpi. All data are means ± SEM with individual values overlaid; N = 5 mice per group and dpi.
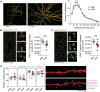
Fig. 4.. Prefrontal microglia deficiency in adolescence induces structural synaptic changes in pyramidal neurons of the adult PFC.
(A) Representative IF stain of a biocytin-filled pyramidal neuron reconstructed with Imaris image analysis software (left), with digitally applied concentric spheres (20-μm spacing) from the soma center (right). The line plot depicts the number of dendritic intersections against the radial distance from soma. *P < 0.05, based on post hoc tests following ANOVA [treatment × distance interaction: F(15,180) = 2.7, P < 0.01]; N = 7 mice per group. (B) Representative double-IF stain using VGLUT1 (red) as presynaptic and PSD-95 (green) as postsynaptic markers of excitatory neurons. VGLUT1+/PSD-95+ colocalizing synapses are highlighted by white circles in magnified sections. The scatterplot shows the density (numbers per square millimeter) of VGLUT1+/PSD-95+ synapses in the medial PFC of PBS (N = 9) and CDS (N = 10) mice. **P < 0.01, t(17) = 2.97. (C) Representative double-IF stain using VGAT (red) as presynaptic and Gephyrin (green) as postsynaptic markers of inhibitory neurons. VGAT+/Gephyrin+ colocalizing synapses are highlighted by white circles in magnified sections. The scatterplot shows the density (numbers per square millimeter) of VGAT+/Gephyrin+ synapses in the PFC of PBS (N = 9) and CDS (N = 10) mice. **P < 0.01, t(17) = 2.88. (D) The photomicrograph shows a representative IF stain of a biocytin-filled pyramidal dendritic section before (top) and after (bottom) surface rendering with Imaris. Different spine classes (1, filopodia; 2, long thin; 3, mushroom; and 4, stubby spines) are highlighted in different colors in the surface-rendered image. The scatterplots show the number of different spines. *P < 0.05, t(12) = 3.22; N = 7 mice per group.
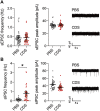
Fig. 5.. Electrophysiological properties of pyramidal neurons in the adult PFC after transient prefrontal microglia depletion in adolescence.
(A) sEPSC frequency and peak amplitude of pyramidal neurons in the adult PFC of PBS and CDS mice. Dots represent individual cells (n = 35 for PBS and n = 27 for CDS; N = 4 mice per group), whereas filled circles reflect cell averages per animal. The picture shows representative sEPSC traces for PBS and CDS mice. (B) sIPSC frequency and peak amplitude of pyramidal neurons in the adult PFC of PBS and CDS mice. Dots represent individual cells (n = 26 cells from N = 3 mice per group), whereas filled circles reflect cell averages per animal. *P < 0.05, t(4) = 4.35, with animals as experimental unit. The picture shows representative sIPSC traces for PBS and CDS mice.
Fig. 6.. Prefrontal microglia deficiency in preadolescence causes selective behavioral and cognitive changes in adulthood.
(A) Phase 1 (D, dummy object; M, unfamiliar mouse) and phase 2 (F, familiar mouse; N, novel mouse) of the social interaction test. The bar plots show the social preference index in phase 1 (values > 0 represent a preference toward M) and social memory index in phase 2 (values > 0 represent a preference toward N), whereas the line plots depict absolute exploration times in either phase. *P < 0.05 [_t_(34) = 2.6]; §_P_ < 0.001 [_F_(1,34) = 46.7], reflecting the overall main effect of object in phase 1; +_P_ < 0.05, reflecting the difference between PBS and CDS mice in terms of the time spent exploring the live mouse, based on post hoc tests following ANOVA [treatment × object interaction: _F_(1,34) = 3.8, _P_ < 0.05]; #_P_ < 0.01, reflecting the overall main effect of object in phase 2 [_F_(1,34) = 8.4]. (**B**) The percentage bar plots depict the relative amount of time (%) exploring the left (L) or right (R) object in sample phases 1 and 2 of the temporal order memory test. The bar plot shows the temporal order memory index during the test phase (values > 0 represent a preference toward the temporally more remote object presented in sample phase 1). (C) Percent time freezing during the habituation, acquisition, and expression phases (left and middle line plots) and extinction rate (% change from freezing levels measured during the expression phase; right line plot) during the contextual fear test. The arrows indicate the presentation of foot shock. *P < 0.05 and **P < 0.01, based on post hoc tests following repeated-measures ANOVA [treatment × days interaction: F(3,102) = 3.2, P < 0.05]. All data are means ± SEM with individual values overlaid; N = 18 male mice in each group and test.
Fig. 7.. Restricted structural synaptic changes in pyramidal neurons of the adult PFC after transient prefrontal microglia deficiency in preadolescence.
(A) The line plot depicts the number of dendritic intersections against the radial distance from soma (20-μm spacing from the soma center), as analyzed by Sholl analysis of biocytin-filled prefrontal neurons reconstructed with Imaris image analysis software. N = 8 per group. (B) Number of different spines (filopodia, long-thin, mushroom, and stubby spines), as analyzed by quantification of surface-rendered spines on biocytin-filled pyramidal dendritic sections. Different spine classes are highlighted in different colors, as shown on the representative IF stain of a biocytin-filled pyramidal dendritic section after surface rendering with Imaris. *P < 0.05, t(14) = 2.66; N = 8 per group. (C) Representative double-IF stain using VGLUT1 (red) as presynaptic and PSD-95 (green) as postsynaptic markers of excitatory neurons. VGLUT1+/PSD-95+ colocalizing synapses are highlighted by white circles in the magnified section. The scatterplot shows the density (numbers per square millimeter) of VGLUT1+/PSD-95+ synapses in the medial PFC of PBS and CDS mice. N = 7 per group. (D) Representative double-IF stain using VGAT (red) as presynaptic and Gephyrin (green) as postsynaptic markers of inhibitory neurons. VGAT+/Gephyrin+ colocalizing synapses are highlighted by white circles in the magnified section. The scatterplot shows the density (numbers per square millimeter) of VGAT+/Gephyrin+ synapses in the PFC of PBS and CDS mice N = 7 per group.
Similar articles
- Prefrontal microglia deficiency during adolescence disrupts adult cognitive functions and synaptic structures: A follow-up study in female mice.
von Arx AS, Dawson K, Lin HY, Mattei D, Notter T, Meyer U, Schalbetter SM. von Arx AS, et al. Brain Behav Immun. 2023 Jul;111:230-246. doi: 10.1016/j.bbi.2023.04.007. Epub 2023 Apr 24. Brain Behav Immun. 2023. PMID: 37100210 - MK-801 Exposure during Adolescence Elicits Enduring Disruption of Prefrontal E-I Balance and Its Control of Fear Extinction Behavior.
Flores-Barrera E, Thomases DR, Tseng KY. Flores-Barrera E, et al. J Neurosci. 2020 Jun 17;40(25):4881-4887. doi: 10.1523/JNEUROSCI.0581-20.2020. Epub 2020 May 19. J Neurosci. 2020. PMID: 32430298 Free PMC article. - Reorganization of adolescent prefrontal cortex circuitry is required for mouse cognitive maturation.
Pöpplau JA, Schwarze T, Dorofeikova M, Pochinok I, Günther A, Marquardt A, Hanganu-Opatz IL. Pöpplau JA, et al. Neuron. 2024 Feb 7;112(3):421-440.e7. doi: 10.1016/j.neuron.2023.10.024. Epub 2023 Nov 17. Neuron. 2024. PMID: 37979584 Free PMC article. - Mechanisms contributing to prefrontal cortex maturation during adolescence.
Caballero A, Granberg R, Tseng KY. Caballero A, et al. Neurosci Biobehav Rev. 2016 Nov;70:4-12. doi: 10.1016/j.neubiorev.2016.05.013. Epub 2016 May 24. Neurosci Biobehav Rev. 2016. PMID: 27235076 Free PMC article. Review. - The role of dopamine and endocannabinoid systems in prefrontal cortex development: Adolescence as a critical period.
Peters KZ, Naneix F. Peters KZ, et al. Front Neural Circuits. 2022 Nov 1;16:939235. doi: 10.3389/fncir.2022.939235. eCollection 2022. Front Neural Circuits. 2022. PMID: 36389180 Free PMC article. Review.
Cited by
- Synapse-specific roles for microglia in development: New horizons in the prefrontal cortex.
Blagburn-Blanco SV, Chappell MS, De Biase LM, DeNardo LA. Blagburn-Blanco SV, et al. Front Mol Neurosci. 2022 Aug 8;15:965756. doi: 10.3389/fnmol.2022.965756. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36003220 Free PMC article. Review. - Early life stress impairs synaptic pruning in the developing hippocampus.
Dayananda KK, Ahmed S, Wang D, Polis B, Islam R, Kaffman A. Dayananda KK, et al. Brain Behav Immun. 2023 Jan;107:16-31. doi: 10.1016/j.bbi.2022.09.014. Epub 2022 Sep 26. Brain Behav Immun. 2023. PMID: 36174883 Free PMC article. - Microglial cannabinoid receptor type 1 mediates social memory deficits in mice produced by adolescent THC exposure and 16p11.2 duplication.
Hasegawa Y, Kim J, Ursini G, Jouroukhin Y, Zhu X, Miyahara Y, Xiong F, Madireddy S, Obayashi M, Lutz B, Sawa A, Brown SP, Pletnikov MV, Kamiya A. Hasegawa Y, et al. Nat Commun. 2023 Oct 25;14(1):6559. doi: 10.1038/s41467-023-42276-5. Nat Commun. 2023. PMID: 37880248 Free PMC article. - Stress-induced reduction of Na+/H+ exchanger isoform 1 promotes maladaptation of neuroplasticity and exacerbates depressive behaviors.
Li Y, Fan C, Wang C, Wang L, Yi Y, Mao X, Chen X, Lan T, Wang W, Yu SY. Li Y, et al. Sci Adv. 2022 Nov 11;8(45):eadd7063. doi: 10.1126/sciadv.add7063. Epub 2022 Nov 11. Sci Adv. 2022. PMID: 36367929 Free PMC article. - Quantitative 3D histochemistry reveals region-specific amyloid-β reduction by the antidiabetic drug netoglitazone.
Catto F, Dadgar-Kiani E, Kirschenbaum D, Economides A, Reuss AM, Trevisan C, Caredio D, Mirzet D, Frick L, Weber-Stadlbauer U, Litvinov S, Koumoutsakos P, Hyung Lee J, Aguzzi A. Catto F, et al. bioRxiv [Preprint]. 2024 Aug 17:2024.08.15.608042. doi: 10.1101/2024.08.15.608042. bioRxiv. 2024. PMID: 39185170 Free PMC article. Preprint.
References
- Koechlin E., Ody C., Kouneiher F., The architecture of cognitive control in the human prefrontal cortex. Science 302, 1181–1185 (2003). - PubMed
- Carlén M., What constitutes the prefrontal cortex? Science 358, 478–482 (2017). - PubMed
- Chini M., Hanganu-Opatz I. L., Prefrontal cortex development in health and disease: Lessons from rodents and humans. Trends Neurosci. 44, 227–240 (2021). - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous