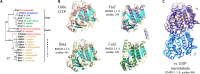Structure and dynamics of Odinarchaeota tubulin and the implications for eukaryotic microtubule evolution - PubMed (original) (raw)
. 2022 Mar 25;8(12):eabm2225.
doi: 10.1126/sciadv.abm2225. Epub 2022 Mar 25.
Samson Ali 1 3, Linh T Tran 1, Jérémie Gaillard 4, Wenfei Li 5, Kenichi Hayashida 6, Mika Hirose 7, Takayuki Kato 7, Atsunori Oshima 6 8 9, Kosuke Fujishima 2 10, Laurent Blanchoin 4 11, Akihiro Narita 3, Robert C Robinson 1 12
Affiliations
- PMID: 35333570
- PMCID: PMC8956254
- DOI: 10.1126/sciadv.abm2225
Structure and dynamics of Odinarchaeota tubulin and the implications for eukaryotic microtubule evolution
Caner Akıl et al. Sci Adv. 2022.
Abstract
Tubulins are critical for the internal organization of eukaryotic cells, and understanding their emergence is an important question in eukaryogenesis. Asgard archaea are the closest known prokaryotic relatives to eukaryotes. Here, we elucidated the apo and nucleotide-bound x-ray structures of an Asgard tubulin from hydrothermal living Odinarchaeota (OdinTubulin). The guanosine 5'-triphosphate (GTP)-bound structure resembles a microtubule protofilament, with GTP bound between subunits, coordinating the "+" end subunit through a network of water molecules and unexpectedly by two cations. A water molecule is located suitable for GTP hydrolysis. Time course crystallography and electron microscopy revealed conformational changes on GTP hydrolysis. OdinTubulin forms tubules at high temperatures, with short curved protofilaments coiling around the tubule circumference, more similar to FtsZ, rather than running parallel to its length, as in microtubules. Thus, OdinTubulin represents an evolutionary stage intermediate between prokaryotic FtsZ and eukaryotic microtubule-forming tubulins.
Figures
Fig. 1.. The crystal structure of OdinTubulin.
(A) Phylogenetic analysis of OdinTubulin from structure-based sequence alignment in comparison to the prokaryotic cell division proteins, FtsZ and CetZ, and the eukaryotic microtubule-forming tubulins. (B) Comparison of the protomer structures of GTP-bound OdinTubulin (PDB 7EVB) to β-tubulin (PDB 6o2r) (19), CetZ (PDB 4b45) (2), and FtsZ (PDB 1w5a) (4). The matching numbers of residues and RMSD values indicate the relative structural similarities to OdinTubulin. RMSD, root mean square deviation. (C) Superimposition of the two GTP-bound OdinTubulin symmetry-related subunits from the crystal packing (dark blue) onto two subunits of eukaryotic tubulin from the GDP-bound microtubule (PDB 6o2r) (19).
Fig. 2.. Structural implications for GTP hydrolysis.
(A) The OdinTubulin protofilament in the crystal packing (PDB 7EVB). Two subunits of OdinTubulin are depicted. The α7 helix and preceding loop (dark blue) and α8 helix and preceding loop (red) comprise the nucleotide sensor motif, which connect the upper and lower GTP-binding sites (sticks). Secondary structure elements are colored by domain: N-terminal (pink), intermediate (cyan), and C-terminal (orange). The nucleotide sensor motif lies within the intermediate domain (see movie S4). (B) Enlargement of the GTP interactions. Only part of each nucleotide sensor motif is shown for clarity. Selected residues from the upper and lower subunits are labeled in yellow and pink, respectively. (C) Enlargement of the interactions around the GTP γ-phosphate. Black, lime green, and cyan spheres indicate Na+, Mg2+ (numbered in black), and water molecules, respectively. The proposed hydrolytic water is shown as a red sphere and labeled “c,” and water molecule suitably placed to receive the hydrogen ion from the hydrolytic water is labeled “h” in blue. The purple dashed line indicates the route for nucleophilic attack on the GTP γ-phosphate (see movie S5). (D) The same region from the GDP-bound structure (PDB 7EVE). Three water molecules (purple) replace the GTP γ-phosphate, and both cations are assigned as Na+ on the basis of bond distances and crystallization conditions (see movie S5). (E to G) Superimposition of protomer structures. (E) GTP-bound OdinTubulin (gray, PDB 7EVB) overlaid on the constrained GDP-bound structure (colored, PDB 7EVE). (F) The unconstrained GDP-bound OdinTubulin (brown, PDB 7F1B) overlaid on the apo structure (colored, PDB 7EVG). (G) GTP-bound OdinTubulin (gray, PDB 7EVB) overlaid on the apo structure (colored, PDB 7EVG). The arrow highlights the conformational change for the intermediate domain (see movie S7).
Fig. 3.. GTP hydrolysis.
(A) Proposed mechanism of GTP hydrolysis. Left: Structure 7EVI is shown with the residues and ions that stabilize the GTP γ-phosphate. c, hydrolytic water; h, hydrogen ion receiving water; m, magnesium ion; k, potassium or sodium ion. Middle: To initiate hydrolysis, the hydrolytic water (orange) is required to approach the GTP γ-phosphate phosphorus atom, along the orange dashed line, likely losing a hydrogen ion to the hydrogen ion receiving water (pink), indicated by the arrow. Right: The dissociated γ-phosphate ion will receive a hydrogen ion, possibly from a surrounding water molecule or from Thr143. (B to E) GTP hydrolysis followed by x-ray crystallography. Structures determined (B) 1 or (C) 72 hours after soaking with 10 mM GTP showed a decrease in the GTP:GDP ratio. (D) Back soaking the crystals (70 hours) decreased the ratio further. (E) Structure of a nonsoaked crystal with 100% GDP bound to OdinTubulin arranged in the regular protofilament packing, similar to the microtubule, which is stabilized by a different crystal unit cell (figs. S1 and S2). The maps are contoured at the color-coded sigma levels. Left: The structures were refined with GDP. Middle: Refined with GTP. Right: The structures were refined with final GTP/GDP ratios. Green (+) and red (−) _F_o-_F_c density indicates the need for more or less atoms, respectively. The arrows indicate the position of two ordered water molecules (purple) that appear following γ-phosphate release after hydrolysis (E, right). The third ordered water molecule (Fig. 2D), which appears after hydrolysis, is bound to the metal ions, occupies a similar position to an oxygen from the GTP γ-phosphate, and does not appear in the difference maps. This water has weaker electron density compared with the other two waters and likely has partial occupancy (movie S5).
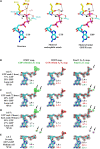
Fig. 4.. Similarities in OdinTubulin and microtubule nucleotide interactions.
(A) Conservation in the sequence of the nucleotide sensor motif from OdinTubulin and human α- and β-tubulins. Colored stars below the alignment indicate the residues highlighted in Fig. 2 (B to D) and in (B) to (E). (B) A water molecule is found bound to α-tubulin Glu254 in the sequestered α/β-tubulin dimer (PDB 6s8k), equivalent to (C) the hydrolytic water “c” bound to Glu251 in OdinTubulin. The 2_F_o-_F_c electron density maps contoured at 1 σ (pink) and the density around potential hydrolytic water (cyan). (D) Subunits within a GDP-bound microtubule E-site (PDB 6o2r) in a similar conformation to Fig. 2B showing structural similarity. (E) β-Tubulin interactions with bound-GTP in the α-tubulin N-site (PDB 6s8k) in a similar orientation to Fig. 2C. The cation-bound, hydrolysis-guiding residue Glu251 from OdinTubulin is substituted by a basic residue (Lys254, indicated by the arrow) in β-tubulin. (F) Coordination of metal ions in molecular dynamics (MD) simulations at the GTP exchangeable site of a microtubule for a representative simulation. A magnesium ion is stably coordinated via oxygen atoms from GTP β-phosphate (cyan) and γ-phosphate (blue) throughout the 1-μs simulation. K+ becomes associated with an oxygen atom from the GTP γ-phosphate (black) and Glu254 (red). Similar results were obtained when Na+ became coordinated at the same site. (G) Occupancies of the metal ions at each site during the simulation. Stable occupancies of the GTP-bound Mg2+ and K+ or Na+ were observed. Approximately half the time, the K+ or Na+ was jointly coordinated by the GTP γ-phosphate and Glu254 (Na+/K+ bridge). Last, a water molecule was located at position “c” between Glu254 and the phosphorous atom of the GTP γ-phosphate (water “c”-bridge).
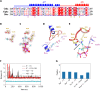
Fig. 5.. Polymerization of OdinTubulin.
(A to F) IRM. (A) Elongation of tubulin (15 μM) into microtubules in 100 mM K-Pipes (pH 6.9), 0.5 mM MgSO4, 0.5 mM EGTA, 10% glycerol, and 0.7 mM GTP. (B to D) Polymerization of OdinTubulin at 6, 2, and 1 μM, respectively, under the same solution conditions. (E) Polymerization of OdinTubulin (0.5 μM) in 100 mM K-Pipes (pH 6.9), 100 mM NaCl, 0.5 mM EGTA, and 0.7 mM GTP or (F) supplemented with 0.5 mM MgSO4. Scale bar, 5 μm. (G to L) EM of negatively stained samples. Two morphologies of filaments were observed. Bundles of straight filaments appeared in solutions of monovalent cations. (G) 100 mM NaCl, 2 mM EGTA with background K+, or (H) 100 mM KCl and 2 mM EGTA. Tubules were observed in the presence of divalent cations. (I) 100 mM NaCl, 2 mM MgSO4, and 2 mM EGTA with background K+. Bundled straight filaments were occasionally observed under these conditions (not shown). The temperature dependence of tubule assembly was monitored in 100 mM KCl, 2 mM MgSO4, and 2 mM EGTA. (J) 4°C, (K) 37°C, and (L) 80°C. Scale bar, 100 nm. (M) Light scattering profiles for native and H393D mutant OdinTubulin (8 μM) on polymerization. GTP (0.7 mM) control (cyan), H393D without (purple) or with GTP (mustard), and native OdinTubulin without (lilac) or with GTP at 30°C (pink) or 37°C (orange). A.U., arbitrary units.
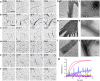
Fig. 6.. OdinTubulin tubule architecture.
(A) OdinTubulin (8 μM) polymerization-monitored light scattering at different temperatures. (B) Cryo–electron micrograph of OdinTubulin (40 or 60 μM) polymerized at 37°C and (C) at 80°C, respectively. Scale bar, 100 nm. (D) Two orientations of the 3D reconstruction at 3-nm resolution of OdinTubulin polymerized at 37°C. (E) The crystal structure fitted into the reconstruction. (F) Two orientations of the 3D reconstruction at 4-nm resolution of OdinTubulin polymerized at 80°C and (G) with the fitted model. (H) Two views of the eukaryotic microtubule (11). Scale bars, 25 nm (D to H).
Fig. 7.. Microtubule adaptations in tubulin.
Structural superimposition of two OdinTubulin subunits (green and gray) onto two laterally related microtubule subunits: (A) β-tubulins (orange and yellow) or (B) α-tubulins (pink and yellow). The microtubule inserts 280 to 282 (blue), relative to OdinTubulin, create a protrusion used for interprotofilament contacts, centered around Tyr283 and His283 in β- and α-tubulins, respectively. The second α-tubulin inserts 365 to 372 (red) interact with the nucleotide sensor motif, indicated by an asterisk. (C and D) Cartoons depicting how the straight-to-curved protofilament transition is used in the two tubule systems. (C) OdinTubulin polymerizes as straight protofilaments, which curve on GTP hydrolysis. The curved protofilaments assemble into tubules. End on view. (D) α/β-Tubulin heterodimers assemble as tubules of typically 13 protofilaments (5 are shown). GTP hydrolysis in the central region of the tubule causes strain; however, the microtubule architecture prevents curving. GTP hydrolysis at the microtubule + ends leads to curving and dissociation, via the loss of curved stretches of protofilaments or monomers. GTP hydrolysis occurs in alternate subunits, reducing the strain and cooperativity within microtubule protofilaments, relative to OdinTubulin, leading to longer more stable protofilaments in the straight form. Side view.
Similar articles
- Bacterial Tubulins A and B Exhibit Polarized Growth, Mixed-Polarity Bundling, and Destabilization by GTP Hydrolysis.
Díaz-Celis C, Risca VI, Hurtado F, Polka JK, Hansen SD, Maturana D, Lagos R, Mullins RD, Monasterio O. Díaz-Celis C, et al. J Bacteriol. 2017 Sep 5;199(19):e00211-17. doi: 10.1128/JB.00211-17. Print 2017 Oct 1. J Bacteriol. 2017. PMID: 28716960 Free PMC article. - Microtubules: an overview.
Wade RH. Wade RH. Methods Mol Med. 2007;137:1-16. doi: 10.2119/molecular%20medicine-2006-00038. Methods Mol Med. 2007. PMID: 18085218 Review. - Conformational changes in tubulin in GMPCPP and GDP-taxol microtubules observed by cryoelectron microscopy.
Yajima H, Ogura T, Nitta R, Okada Y, Sato C, Hirokawa N. Yajima H, et al. J Cell Biol. 2012 Aug 6;198(3):315-22. doi: 10.1083/jcb.201201161. Epub 2012 Jul 30. J Cell Biol. 2012. PMID: 22851320 Free PMC article. - Straight GDP-tubulin protofilaments form in the presence of taxol.
Elie-Caille C, Severin F, Helenius J, Howard J, Muller DJ, Hyman AA. Elie-Caille C, et al. Curr Biol. 2007 Oct 23;17(20):1765-70. doi: 10.1016/j.cub.2007.08.063. Epub 2007 Oct 4. Curr Biol. 2007. PMID: 17919908 - How tubulin subunits are lost from the shortening ends of microtubules.
Tran PT, Joshi P, Salmon ED. Tran PT, et al. J Struct Biol. 1997 Mar;118(2):107-18. doi: 10.1006/jsbi.1997.3844. J Struct Biol. 1997. PMID: 9126637 Review.
Cited by
- The emerging view on the origin and early evolution of eukaryotic cells.
Vosseberg J, van Hooff JJE, Köstlbacher S, Panagiotou K, Tamarit D, Ettema TJG. Vosseberg J, et al. Nature. 2024 Sep;633(8029):295-305. doi: 10.1038/s41586-024-07677-6. Epub 2024 Sep 11. Nature. 2024. PMID: 39261613 Review. - The eukaryotic-like characteristics of small GTPase, roadblock and TRAPPC3 proteins from Asgard archaea.
Tran LT, Akıl C, Senju Y, Robinson RC. Tran LT, et al. Commun Biol. 2024 Mar 12;7(1):273. doi: 10.1038/s42003-024-05888-1. Commun Biol. 2024. PMID: 38472392 Free PMC article. - The Host Cytoskeleton Functions as a Pleiotropic Scaffold: Orchestrating Regulation of the Viral Life Cycle and Mediating Host Antiviral Innate Immune Responses.
Li M, Peng D, Cao H, Yang X, Li S, Qiu HJ, Li LF. Li M, et al. Viruses. 2023 Jun 12;15(6):1354. doi: 10.3390/v15061354. Viruses. 2023. PMID: 37376653 Free PMC article. Review. - Diverse cytomotive actins and tubulins share a polymerization switch mechanism conferring robust dynamics.
Wagstaff JM, Planelles-Herrero VJ, Sharov G, Alnami A, Kozielski F, Derivery E, Löwe J. Wagstaff JM, et al. Sci Adv. 2023 Mar 29;9(13):eadf3021. doi: 10.1126/sciadv.adf3021. Epub 2023 Mar 29. Sci Adv. 2023. PMID: 36989372 Free PMC article. - Alternative Approaches to Understand Microtubule Cap Morphology and Function.
Oliva MÁ, Gago F, Kamimura S, Díaz JF. Oliva MÁ, et al. ACS Omega. 2023 Jan 13;8(4):3540-3550. doi: 10.1021/acsomega.2c06926. eCollection 2023 Jan 31. ACS Omega. 2023. PMID: 36743020 Free PMC article. Review.
References
- Oliva M. A., Cordell S. C., Lowe J., Structural insights into FtsZ protofilament formation. Nat. Struct. Mol. Biol. 11, 1243–1250 (2004). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources