Whole Genome Transcriptomic Analysis of Ovary Granulosa Cells Revealed an Anti-Apoptosis Regulatory Gene DLGAP5 in Polycystic Ovary Syndrome - PubMed (original) (raw)
doi: 10.3389/fendo.2022.781149. eCollection 2022.
Hu Li 2 3 4, Yi Song 1, Juan Cen 5, Yuying Zhang 6, Yi Sui 7, Dexuan Cui 8, Tin Chiu Li 1, Yan Xu 3, Chi Chiu Wang 1, Pui Wah Jacqueline Chung 1, Tao Tang 1 3
Affiliations
- PMID: 35370991
- PMCID: PMC8971550
- DOI: 10.3389/fendo.2022.781149
Whole Genome Transcriptomic Analysis of Ovary Granulosa Cells Revealed an Anti-Apoptosis Regulatory Gene DLGAP5 in Polycystic Ovary Syndrome
Yan Deng et al. Front Endocrinol (Lausanne). 2022.
Abstract
The mechanisms underlining pathogenesis of polycystic ovary syndrome (PCOS) remain largely unknown. Dysfunction of ovarian granulosa cells plays an important role. The present study performed the lncRNA and mRNA profiling by whole genome transcriptomic sequencing of ovary granulosa cells from women with PCOS and investigated the potential role of differentially expressed gens (DEGs) in the pathomechanism of PCOS. In total, 1,936 DEGs (30 upregulated and 1,906 downregulated mRNAs and lncRNAs) were identified in the ovary granulosa cells between control and PCOS group. Functional enrichment analysis showed that DEGs were mainly associated with cytokine-cytokine receptor interaction, neuroactive ligand-receptor interaction, and olfactory transduction. qRT-PCR validated the upregulation of DLGAP5 mRNA in ovary from PCOS group when compared to control group. Immunostaining and TUNEL assays showed that DLGAP5 protein level was increased while apoptosis was decreased in follicles of ovary in PCOS group. In vitro functional assays showed that DLGPA5 knockdown repressed viability and proliferation, but enhanced apoptosis and disrupted cell cycle in granulosa cells; while DLGAP5 overexpression had the opposite effects in granulosa cells. In conclusion, the study showed differentially expressed lncRNA and mRNA profile in the granulosa cells in ovaries of PCOS. Functional results demonstrated that DLGAP5 is a dysregulated candidate gene in the pathogenesis of PCOS, especially granulosa cell apoptosis and proliferation.
Keywords: DLGAP5; PCOS; apoptosis; ovary granulosa cells; transcriptomic analysis.
Copyright © 2022 Deng, Li, Song, Cen, Zhang, Sui, Cui, Li, Xu, Wang, Chung and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Figure 1
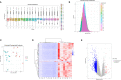
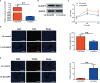
RNA-seq analysis of differentially expressed genes between PCOS and control group. (A) Boxplots of abundance of transcripts in each sample. (B) RPKM density distribution in each sample. (C) PCA of averaged, rank-normalized read counts from RNA-seq. (D) Heatmap analysis of differentially expressed genes between control group and PCOS group. (E) Volcano plots showing the significance and log2 fold-change (logFC) for all gene transcripts reliably detected in the RNA-seq analysis between control group and PCOS group.
Figure 2
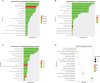
Functional enrichment analysis of differentially expressed genes. (A) GO enrichment analysis of DEGs in biological process. (B) GO enrichment analysis of DEGs in cellular component. (C) GO enrichment analysis of DEGs in molecular function. (D) KEGG enrichment analysis of DEGs. DEGs, differentially expressed genes.
Figure 3
Alternative splicing analysis. (A) Alternative splicing types. A3SS, alternative 3’ splice site; A5SS, alternative 5’ splice site; MXE, mutually exclusive exons; RI, Retained Intron; SE, skipped exon. (B) Alternative splicing number. (C) GO enrichment analysis of alternative spliced genes in cellular component. (D) GO enrichment analysis of alternative spliced genes in molecular function. (E) GO enrichment analysis of alternative spliced genes in molecular function. (F) KEGG enrichment analysis of alternative spliced genes.
Figure 4
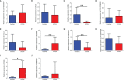
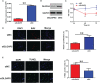
Validation analysis of selected differentially expressed candidate genes between control group and PCOS group. The mRNA expression levels of (A) LOC105379507, (B) CXCL8, (C) AREG, (D) LOC107986562, (E) CXCL2, (F) DLGAP5, (G) Lin001778, (H) LOC105379355, (I) PADI6, and (J) SNRA9 in the ovarian tissues between control group and PCOS group. N = 8–11. *P < 0.05 and **P < 0.01.
Figure 5
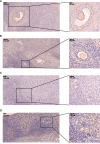
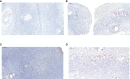
Immunostaining analysis of DLGAP5 in the ovarian tissues. (A) Immunostaining of DLGAP5 in control group. Scale bar = 200 µm. (B) Immunostaining of DLGAP5 in PCOS group. Scale bar = 200 µm. (C) Immunostaining of DLGAP5 in control group. Scale bar = 200 µm. (D) Immunostaining of DLGAP5 in PCOS group. Scale bar = 200 µm. Blue arrows indicate primary or antral follicles.
Figure 6
TUNEL analysis of ovarian tissues in the normal group and PCOS group. (A, C) TUNEL staining of ovarian tissues in control group. Scale bar = 200 µm. (B, D) TUNEL staining of ovarian tissues in PCOS group. Scale bar = 200 µm. Scale bar = 200 µm. Red arrows indicate primary, secondary or antral follicles.
Figure 7
Effects of DLGAP5 knockdown on the granulosa cell proliferation and apoptosis. Granulosa cells were transfected with si-NC or si-DLGAP5, and after siRNA transfection, (A) the mRNA expression level of DLGAP5 in the granulosa cells was determined by qRT-PCR; (B) the protein expression level of DLGAP5 in the granulosa cells was determined by western blot; (C) the cell viability of granulosa cells was determined by CCK-8 assay; (D) the cell proliferation of granulosa cells was determined by EdU assay, scale bar = 50 µm; (E) the cell apoptosis of granulosa cells was determined by TUNEL assay; scale bar = 50 µm. N = 3. **P < 0.01.
Figure 8
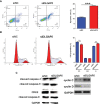
Effects of DLGAP5 knockdown on the granulosa cell apoptosis and cell cycle. Granulosa cells were transfected with si-NC or si-DLGAP5, and after siRNA transfection, (A) the cell apoptotic rates and (B) cell cycle of granulosa cells were determined by flow cytometry; (C) the protein levels of cleaved caspase-3, cleaved caspase-9, CDK2, cleaved caspase-8, cyclin C and cyclin D in granulosa cells were determined by Western blot assay. N = 3. **P < 0.01 and ***P < 0.001.
Figure 9
Effects of DLGAP5 overexpression on the granulosa cell proliferation and apoptosis. Granulosa cells were transfected with LV-control or LV-DLGAP5, and after transfection, (A) the mRNA expression level of DLGAP5 in the granulosa cells was determined by qRT-PCR; (B) the protein expression level of DLGAP5 in the granulosa cells was determined by western blot; (C) the cell viability of granulosa cells was determined by CCK-8 assay; (D) the cell proliferation of granulosa cells was determined by EdU assay, scale bar = 100 µm; (E) the cell apoptosis of granulosa cells was determined by TUNEL assay, scale bar = 100 µm. *P < 0.05 and **P < 0.01.
Figure 10
Effects of DLGAP5 overexpression on the granulosa cell apoptosis and cell cycle. Granulosa cells were transfected with LV-control or LV-DLGAP5, and after transfection, (A) the cell apoptotic rates and (B) cell cycle of granulosa cells were determined by flow cytometry; (C) the protein levels of cleaved caspase-3, cleaved caspase-9, CDK2, cleaved caspase-8, cyclin C and cyclin D in granulosa cells were determined by Western blot assay. *P < 0.05.
Similar articles
- Whole transcriptome analysis and construction of gene regulatory networks of granulosa cells from patients with polycystic ovary syndrome (PCOS).
Yuan Y, Daiterigele, Zhang Q, Du C. Yuan Y, et al. Eur J Med Res. 2025 Jan 7;30(1):9. doi: 10.1186/s40001-024-02237-0. Eur J Med Res. 2025. PMID: 39773546 Free PMC article. - Functional microarray analysis of differentially expressed genes in granulosa cells from women with polycystic ovary syndrome related to MAPK/ERK signaling.
Lan CW, Chen MJ, Tai KY, Yu DC, Yang YC, Jan PS, Yang YS, Chen HF, Ho HN. Lan CW, et al. Sci Rep. 2015 Oct 13;5:14994. doi: 10.1038/srep14994. Sci Rep. 2015. PMID: 26459919 Free PMC article. - Downregulating lncRNA NEAT1 induces proliferation and represses apoptosis of ovarian granulosa cells in polycystic ovary syndrome via microRNA-381/IGF1 axis.
Zhen J, Li J, Li X, Wang X, Xiao Y, Sun Z, Yu Q. Zhen J, et al. J Biomed Sci. 2021 Jul 15;28(1):53. doi: 10.1186/s12929-021-00749-z. J Biomed Sci. 2021. PMID: 34266430 Free PMC article. Retracted. - Programmed cell death 4: A novel player in the pathogenesis of polycystic ovary syndrome.
Zarezadeh R, Abbasi K, Aboutalebi Vand Beilankouhi E, Navali N, Hakimi P, Fattahi A, Farzadi L. Zarezadeh R, et al. Cell Biochem Funct. 2024 Jan;42(1):e3905. doi: 10.1002/cbf.3905. Epub 2023 Dec 19. Cell Biochem Funct. 2024. PMID: 38115175 Review. - The Roles of Autophagy in the Genesis and Development of Polycystic Ovary Syndrome.
Cheng D, Zheng B, Sheng Y, Zeng Z, Mo Z. Cheng D, et al. Reprod Sci. 2023 Oct;30(10):2920-2931. doi: 10.1007/s43032-023-01255-3. Epub 2023 May 19. Reprod Sci. 2023. PMID: 37204635 Review.
Cited by
- Granulosa cell insight: unraveling the potential of menstrual blood-derived stem cells and their exosomes on mitochondrial mechanisms in polycystic ovary syndrome (PCOS).
Mansoori M, Solhjoo S, Palmerini MG, Nematollahi-Mahani SN, Ezzatabadipour M. Mansoori M, et al. J Ovarian Res. 2024 Aug 17;17(1):167. doi: 10.1186/s13048-024-01484-3. J Ovarian Res. 2024. PMID: 39153978 Free PMC article. - miR-34a-5p modulation of polycystic ovary syndrome via targeting the NOTCH signaling pathway.
Zhang K, Wang X, Liu F, Lin H, Wang Y, Zhao M, Wang X, Chu Y, Xu L. Zhang K, et al. J Ovarian Res. 2025 Mar 13;18(1):55. doi: 10.1186/s13048-025-01623-4. J Ovarian Res. 2025. PMID: 40082891 Free PMC article. - LncRNA NEAT1 and MALAT1 are involved in polycystic ovary syndrome pathogenesis by functioning as competing endogenous RNAs to control the expression of PCOS-related target genes.
ElMonier AA, El-Boghdady NA, Fahim SA, Sabry D, Elsetohy KA, Shaheen AA. ElMonier AA, et al. Noncoding RNA Res. 2023 Mar 3;8(2):263-271. doi: 10.1016/j.ncrna.2023.02.008. eCollection 2023 Jun. Noncoding RNA Res. 2023. PMID: 36935861 Free PMC article. - Whispers of the polycystic ovary syndrome theater: Directing role of long noncoding RNAs.
Lin X, Nie X, Deng P, Wang L, Hu C, Jin N. Lin X, et al. Noncoding RNA Res. 2024 May 14;9(4):1023-1032. doi: 10.1016/j.ncrna.2024.05.003. eCollection 2024 Dec. Noncoding RNA Res. 2024. PMID: 39022674 Free PMC article. Review.
References
- Chung PW, Chan SS, Yiu KW, Lao TT, Chung TK. Menstrual Disorders in a Paediatric and Adolescent Gynaecology Clinic: Patient Presentations and Longitudinal Outcomes. Hong Kong Med J = Xianggang Yi Xue Za Zhi (2011) 17:391–7. - PubMed
- Ovesen PG, Moller N, Greisen S, Ingerslev HJ. [Polycystic Ovary Syndrome Ii. Endocrinol Metabolism] Ugeskrift laeger (1998) 160:265–9. - PubMed
- Kong GW, Cheung LP, Lok IH. Effects of Laparoscopic Ovarian Drilling in Treating Infertile Anovulatory Polycystic Ovarian Syndrome Patients With and Without Metabolic Syndrome. Hong Kong Med J = Xianggang Yi Xue Za Zhi (2011) 17:5–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous