Epidermolysis Bullosa With Congenital Absence of Skin: Congenital Corneal Cloudiness and Esophagogastric Obstruction Including Extended Genotypic Spectrum of PLEC, LAMC2, ITGB4 and COL7A1 - PubMed (original) (raw)
doi: 10.3389/fgene.2022.847150. eCollection 2022.
Sanchawan Wittayakornrerk 1, Ramrada Lekwuttikarn 1, Sasikarn Pakdeeto 2, Piangor Watcharakuldilok 3, Chatchay Prempunpong 1, Thipwimol Tim-Aroon 1, Chawintee Puttanapitak 4, Piyawan Wattanasoontornsakul 5, Thitiporn Junhasavasdikul 6, Parith Wongkittichote 1 7, Saisuda Noojarern 1, Duangrurdee Wattanasirichaigoon 1
Affiliations
- PMID: 35432467
- PMCID: PMC9010945
- DOI: 10.3389/fgene.2022.847150
Epidermolysis Bullosa With Congenital Absence of Skin: Congenital Corneal Cloudiness and Esophagogastric Obstruction Including Extended Genotypic Spectrum of PLEC, LAMC2, ITGB4 and COL7A1
Pharuhad Pongmee et al. Front Genet. 2022.
Abstract
Epidermolysis bullosa (EB) is a rare and genetically heterogeneous disorder characterized by skin fragility and blister formation occurring spontaneously or after minor trauma. EB is accompanied by congenital absence of skin (EB with CAS) in some patients. Pathogenic variants of COL7A1 are responsible for EB with CAS in the vast majority of cases. Type and subtype diagnosis of EB with CAS generally requires specific immunohistological examinations that are not widely available plus targeted gene analysis. The present study aimed to determine the clinical features of five patients affected by EB with CAS and to identify the underlying genetic defects using whole exome sequencing (WES) followed by focused analysis of the target genes. Four patients had generalized skin involvement and one had localized defects. Two patients exhibited extremely severe skin manifestations and congenital cloudy cornea along with pyloric atresia, and one had partial esophagogastric obstruction and anuria due to vesicoureteric obstruction. In the WES analysis, the average coverage of the target exons was 99.05% (726 of 733 exons), with a range of 96.4-100% for individual genes. We identified four novel and two known pathogenic/likely pathogenic variants of five distinct genes in the examined families: PLEC:c.2536G > T (p.Glu846Ter); LAMC2:c.3385C > T (p.Arg1129Ter); KRT5:c.429G > A (p.Glu477Lys); _ITGB4:_c.794dupC (p.Ala266SerfsTer5); COL7A1:c.5440C > T (p.Arg1814Cys); and COL7A1:c.6103delG. All alleles were inherited from the parents, except for the KRT5 variant as a de novo finding. The findings reveal extremely rare phenotypes found in EB with CAS, namely congenital cloudy cornea, esophagogastric obstruction, and anuria, and extend the genotypic spectrum of EB-related genes. The data confirm that WES provides very high coverage of coding exons/genes and support its use as a reasonable alternative method for diagnosis of EB. The present data from an underrepresented population in Southeast Asia could further broaden the knowledge and research on EB.
Keywords: bilateral hydronephrosis; cloudy cornea; epidermolysis bullosa with pyloric atresia; intestinal obstruction; reflux nephropathy.
Copyright © 2022 Pongmee, Wittayakornrerk, Lekwuttikarn, Pakdeeto, Watcharakuldilok, Prempunpong, Tim-Aroon, Puttanapitak, Wattanasoontornsakul, Junhasavasdikul, Wongkittichote, Noojarern and Wattanasirichaigoon.
Conflict of interest statement
The authors declare that the research was constructed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
FIGURE 1
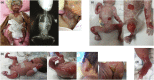
Characteristics of skin involvement and other abnormalities of Patients 1–3 and 5. (A) Patient 1: marked multiple absence of skin and well-demarcated erythematous atrophic patches on the face, neck, upper chest wall, and upper and lower extremities; and x-ray showing single large gastric bubble suggesting pyloric atresia. (B) Patient 2: well-demarcated erythematous atrophic patches and absence of skin on both legs, extending to the feet, and tense large bullae at the posterior aspect of left ear pinna, sacral area, and buttocks. (C) Patient 3: notable well-defined erythematous atrophic patches and absence of skin at the abdominal wall, forearms, and both legs. (D) Patient 5: localized absence of skin at the dorsum of the right foot up to the ankle.
FIGURE 2
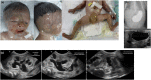
Characteristics of skin involvement and other abnormalities of Patient 4. (A) Skin involvement: marked complete absence of skin over the parietotemporal region of the scalp and extending downward to the interorbital and midfacial areas including the nasal region; perineal area; upper and lower extremities including thighs, legs, feet, arms, forearms, and hands; bilateral cloudy cornea, ectropion of the left lower eyelid and dysplastic ears. (B) Pyloric atresia: an upper gastrointestinal study showing a gastric outlet obstruction and abdominal ultrasound showing large air-filled stomach. (C) Abdominal ultrasound demonstrating multi-leveled urinary tract obstructions: severe hydronephrosis of the left kidney (far left); left ureteropelvic junction obstruction (middle); and a small and collapsed urinary bladder and markedly dilated left ureter, signifying a vesicoureteric junction obstruction (far right). Similar findings were observed on the right kidney and ureter (data not shown).
FIGURE 3
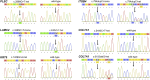
Chromatogram confirming the sequences of the mutations in PLEC, LAMC2, KRT5, ITGB4, and COL7A1 identified in the study.
FIGURE 4
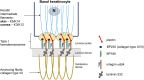
Schematic diagram showing basal keratinocytes and type I hemidesmosomes in the skin and corneal epithelium. Keratin intermediate filaments form within the basal layer. Plectin, a hemidesmosomal protein, directly binds to the intermediate filaments and integrin α6β4, the latter interacts with BP230 and BP180. Laminin-332 binds to integrin α6β4 and the anchoring fibrils composed of collagen type VII.
Similar articles
- Case report: A case of epidermolysis bullosa complicated with pyloric atresia and a literature review.
Luo C, Yang L, Huang Z, Su Y, Lu Y, Yu D, Zhang M, Wu K. Luo C, et al. Front Pediatr. 2023 Mar 23;11:1098273. doi: 10.3389/fped.2023.1098273. eCollection 2023. Front Pediatr. 2023. PMID: 37033187 Free PMC article. - Epidermolysis Bullosa with Pyloric Atresia.
Lucky AW, Gorell E. Lucky AW, et al. 2008 Feb 22 [updated 2023 Jan 26]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2024. 2008 Feb 22 [updated 2023 Jan 26]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2024. PMID: 20301336 Free Books & Documents. Review. - Detection of Novel Biallelic Causative Variants in COL7A1 Gene by Whole-Exome Sequencing, Resulting in Congenital Recessive Dystrophic Epidermolysis Bullosa in Three Unrelated Families.
Fozia F, Nazli R, Alrashed MM, Ghneim HK, Haq ZU, Jabeen M, Alam Khan S, Ahmad I, Bourhia M, Aboul-Soud MAM. Fozia F, et al. Diagnostics (Basel). 2022 Jun 23;12(7):1525. doi: 10.3390/diagnostics12071525. Diagnostics (Basel). 2022. PMID: 35885431 Free PMC article. - Mutational analysis of epidermolysis bullosa in Taiwan by whole-exome sequencing complemented by RNA sequencing: a series of 77 patients.
Tu WT, Hou PC, Chen PC, Chen WR, Huang HY, Wang JY, Huang YT, Wu YH, Su CL, Tang YA, Iwata H, Natsuga K, Chao SC, Sun HS, Tang MJ, Lee JY, McGrath JA, Hsu CK. Tu WT, et al. Orphanet J Rare Dis. 2022 Dec 28;17(1):451. doi: 10.1186/s13023-022-02605-1. Orphanet J Rare Dis. 2022. PMID: 36578049 Free PMC article. - An overview of the genetic basis of epidermolysis bullosa in Brazil: discovery of novel and recurrent disease-causing variants.
Mariath LM, Santin JT, Frantz JA, Doriqui MJR, Kiszewski AE, Schuler-Faccini L. Mariath LM, et al. Clin Genet. 2019 Sep;96(3):189-198. doi: 10.1111/cge.13555. Epub 2019 May 29. Clin Genet. 2019. PMID: 31001817 Review.
Cited by
- Shear-Induced ITGB4 Promotes Endothelial Cell Inflammation and Atherosclerosis.
Kong X, Chen S, Luo S, Chen A, Wang L, Tang H, Wang F, Wang Z, Gao X, Zuo G, Zhou W, Gu Y, Ge Z, Zhang J. Kong X, et al. Oxid Med Cell Longev. 2022 Oct 25;2022:5842677. doi: 10.1155/2022/5842677. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36329801 Free PMC article.
References
- Bishnoi P., Ng Y. Z., Wei H., Tan E. C., Lunny D. P., Wong X. F. C. C., et al. (2021). Self‐improving Dystrophic Epidermolysis Bullosa: First Report of Clinical, Molecular, and Genetic Characterization of Five Patients from Southeast Asia. Am. J. Med. Genet. 185, 625–630. 10.1002/ajmg.a.61975 - DOI - PubMed
- Bonduelle M., De Raeve L., Charlesworth A., Gagnoux-palacios L., Ortonne J.-P., Meneguzzi G. (2003). Identification of a Lethal Form of Epidermolysis Bullosa Simplex Associated with a Homozygous Genetic Mutation in Plectin. J. Invest. Dermatol. 121, 1344–1348. 10.1111/j.1523-1747.2003.12639.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous