Population genomics confirms acquisition of drug-resistant Aspergillus fumigatus infection by humans from the environment - PubMed (original) (raw)
doi: 10.1038/s41564-022-01091-2. Epub 2022 Apr 25.
Alireza Abdolrasouli 2 3, Katie Dunne 4, Thomas R Sewell 5, Yuyi Zhang 5, Eloise Ballard 6, Amelie P Brackin 5, Norman van Rhijn 7, Harry Chown 7, Alexandra Tsitsopoulou 8, Raquel B Posso 9, Sanjay H Chotirmall 10, Noel G McElvaney 11, Philip G Murphy 4 12, Alida Fe Talento 4, Julie Renwick 4, Paul S Dyer 13, Adrien Szekely 14, Paul Bowyer 7, Michael J Bromley 7, Elizabeth M Johnson 14 15, P Lewis White 9, Adilia Warris 6 15, Richard C Barton 16, Silke Schelenz 17, Thomas R Rogers 4, Darius Armstrong-James 18, Matthew C Fisher 19
Affiliations
- PMID: 35469019
- PMCID: PMC9064804
- DOI: 10.1038/s41564-022-01091-2
Population genomics confirms acquisition of drug-resistant Aspergillus fumigatus infection by humans from the environment
Johanna Rhodes et al. Nat Microbiol. 2022 May.
Erratum in
- Publisher Correction: Population genomics confirms acquisition of drug-resistant Aspergillus fumigatus infection by humans from the environment.
Rhodes J, Abdolrasouli A, Dunne K, Sewell TR, Zhang Y, Ballard E, Brackin AP, van Rhijn N, Chown H, Tsitsopoulou A, Posso RB, Chotirmall SH, McElvaney NG, Murphy PG, Talento AF, Renwick J, Dyer PS, Szekely A, Bowyer P, Bromley MJ, Johnson EM, Lewis White P, Warris A, Barton RC, Schelenz S, Rogers TR, Armstrong-James D, Fisher MC. Rhodes J, et al. Nat Microbiol. 2022 Nov;7(11):1944. doi: 10.1038/s41564-022-01160-6. Nat Microbiol. 2022. PMID: 36203089 Free PMC article. No abstract available.
Abstract
Infections caused by the fungal pathogen Aspergillus fumigatus are increasingly resistant to first-line azole antifungal drugs. However, despite its clinical importance, little is known about how susceptible patients acquire infection from drug-resistant genotypes in the environment. Here, we present a population genomic analysis of 218 A. fumigatus isolates from across the UK and Ireland (comprising 153 clinical isolates from 143 patients and 65 environmental isolates). First, phylogenomic analysis shows strong genetic structuring into two clades (A and B) with little interclade recombination and the majority of environmental azole resistance found within clade A. Second, we show occurrences where azole-resistant isolates of near-identical genotypes were obtained from both environmental and clinical sources, indicating with high confidence the infection of patients with resistant isolates transmitted from the environment. Third, genome-wide scans identified selective sweeps across multiple regions indicating a polygenic basis to the trait in some genetic backgrounds. These signatures of positive selection are seen for loci containing the canonical genes encoding fungicide resistance in the ergosterol biosynthetic pathway, while other regions under selection have no defined function. Lastly, pan-genome analysis identified genes linked to azole resistance and previously unknown resistance mechanisms. Understanding the environmental drivers and genetic basis of evolving fungal drug resistance needs urgent attention, especially in light of increasing numbers of patients with severe viral respiratory tract infections who are susceptible to opportunistic fungal superinfections.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
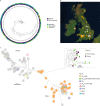
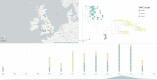
Fig. 1. Phylogeographical and phenotypic variation of 218 A. fumigatus isolates in the UK and Republic of Ireland.
a, Unrooted maximum likelihood phylogenetic tree (constructed in RAxML using genome-wide SNPs) showing the itraconazole MIC breakpoint (defined as above or below 2 mg l−1 for resistance or susceptibility, respectively) and clinical or environmental source of isolation. b, Map showing the location of isolation for isolates included in this study, with the legend (bottom right) indicating the cyp51A polymorphisms present. c, Unrooted maximum likelihood phylogeny of all 218 isolates showing the genetic relationship between isolates. ‘Clade A’ and ‘clade B’ indicate the clustered nature of triazole resistance polymorphisms. The subclade in the midpoint of the phylogeny indicates a clonal clade, clade A_A_, which is rich in clinical and environmental A. fumigatus isolates that contain the drug resistance polymorphism TR34/L98H, highlighted in the inset phylogeny. Source data
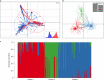
Fig. 2. Occurrence of three subclusters within the A. fumigatus population; clinical and environmental isolates were drawn from a single population.
a, Scatterplot of the PCA of A. fumigatus genotypes using the first two principal components illustrating genetic identity for clinical and environmental isolates. b, Discriminant PCA and PCA broadly identified three clusters, clusters 1–3, corresponding to the lowest BIC. c, Three subclusters were confirmed using STRUCTURE and k = 3. Source data
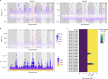
Fig. 3. Loci associated with itraconazole resistance linked to regions of high F_ST_ and selection.
a, Scatterplot of Tajima’s D estimates for each chromosome for all isolates within clade A (left) and scatterplot of Tajima’s D estimates for each chromosome for all isolates within clade B (right). The position of cyp51A is highlighted in red. b, Scatterplot of sliding 10-kb non-overlapping window estimates of F_ST_ for each chromosome between isolates within clades A and B (top). Manhattan plot (bottom) for the treeWAS subsequent test (bottom) showing P values for all loci and a significant threshold of 0.01 (dashed red line), above which points indicate significant associations. The vertical red line in both plots denotes the position of cyp51A. c, Relative growth of null mutants (compared to A1160) of genes with significant loci identified in treeWAS on media containing itraconazole. Source data
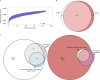
Fig. 4. Statistical analysis of the open pan-genome associates genes from itraconazole-resistant isolates within either clade A or B based on the prevalence of WT cyp51a.
a, Plot of Tettelin’s model on the pan-genome generated from all isolates. The red line indicates the median value from all permutations and the γ value indicates that the pan-genome (γ > 0) is classed as ‘open’. b, Venn diagram plotting genes occurring at least once within isolates from clades A and B and genes only found within isolates from clade A or B. c, Venn diagram plotting genes associated with clades A and B, itraconazole resistance/susceptibility and WT/mutant cyp51a. Associated genes obtained a Bonferroni P < 0.05 and were organized into groups based on log ORs, from the Scoary results. The numbers represent the gene numbers. Source data
Extended Data Fig. 1
Microreact project screenshot of the dataset
https://microreact.org/project/viUDBzrCmTNKmY9Fu6Zhxi
.
Extended Data Fig. 2
Phylogenetic analysis of all 218_A. fumigatus_ isolates with bootstrap support over 1000 replicates performed on WGS SNP data to generate maximum-likelihood phylogeny. Branch lengths represent average number of SNPs.
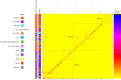
Extended Data Fig. 3. Genome sharing fineStructure analysis of A. fumigatus using genome-wide SNPs confirms the presence of three populations within the dataset.
Population-averaged coancestry matrix for the linked model dataset with associated cyp51A polymorphism and itraconazole MIC (defined as above or below 2 mg l−1 for resistance or susceptibility, respectively, or not done). The right-hand scale bar represents the amount of genomic sharing, with blue/black representing the largest amount of sharing of genetic material and yellow representing the least amount of shared genetic material. Source data
Extended Data Fig. 4
Phylogenetic analysis of all 218 A. fumigatus isolates plus an additional 41 publicly available WGS of non-UK origin confirms the clade assignment into Clades A and B (Supplementary Fig. 6) was not an artifact of these data but also seen globally. These additional data comprised of 33 clinical and 8 environmental isolates. Maximum-likelihood phylogeny generated with bootstrap support over 1000 replicates on WGS SNP data. Source data
Extended Data Fig. 5
Microreact project screenshot of the dataset with DAPC clusters showing lack of geographic and temporal clustering. Source data
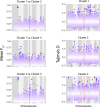
Extended Data Fig. 6
Scatterplots of sliding 10-kb non-overlapping window estimates of F_ST_ for each chromosome between isolates within Clusters 1 and 2, Clusters 1 and 3, and Clusters 2 and 3 (top to bottom, left panel). Scatterplots of Tajima’s D estimates for each chromosome for all isolates within Clusters 1, 2 and 3 (right panel). The position of cyp51A is highlighted in red.
Similar articles
- Effects of Agricultural Fungicide Use on Aspergillus fumigatus Abundance, Antifungal Susceptibility, and Population Structure.
Barber AE, Riedel J, Sae-Ong T, Kang K, Brabetz W, Panagiotou G, Deising HB, Kurzai O. Barber AE, et al. mBio. 2020 Nov 24;11(6):e02213-20. doi: 10.1128/mBio.02213-20. mBio. 2020. PMID: 33234685 Free PMC article. - Genomic Context of Azole Resistance Mutations in Aspergillus fumigatus Determined Using Whole-Genome Sequencing.
Abdolrasouli A, Rhodes J, Beale MA, Hagen F, Rogers TR, Chowdhary A, Meis JF, Armstrong-James D, Fisher MC. Abdolrasouli A, et al. mBio. 2015 Jun 2;6(3):e00536. doi: 10.1128/mBio.00536-15. mBio. 2015. PMID: 26037120 Free PMC article. - High detection rate of azole-resistant Aspergillus fumigatus after treatment with azole antifungal drugs among patients with chronic pulmonary aspergillosis in a single hospital setting with low azole resistance.
Takeda K, Suzuki J, Watanabe A, Arai T, Koiwa T, Shinfuku K, Narumoto O, Kawashima M, Fukami T, Tamura A, Nagai H, Matsui H, Kamei K. Takeda K, et al. Med Mycol. 2021 Apr 6;59(4):327-334. doi: 10.1093/mmy/myaa052. Med Mycol. 2021. PMID: 32642756 - Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms.
Chowdhary A, Sharma C, Hagen F, Meis JF. Chowdhary A, et al. Future Microbiol. 2014;9(5):697-711. doi: 10.2217/fmb.14.27. Future Microbiol. 2014. PMID: 24957095 Review.
Cited by
- A Whole Genome Sequencing-Based Approach to Track down Genomic Variants in Itraconazole-Resistant Species of Aspergillus from Iran.
Nargesi S, Valadan R, Abastabar M, Kaboli S, Thekkiniath J, Hedayati MT. Nargesi S, et al. J Fungi (Basel). 2022 Oct 17;8(10):1091. doi: 10.3390/jof8101091. J Fungi (Basel). 2022. PMID: 36294656 Free PMC article. - Phenotypic Variants of Azole-Resistant Aspergillus Fumigatus that Co-exist in Human Respiratory Samples are Genetically Highly Related.
Abdolrasouli A, Rhodes JL. Abdolrasouli A, et al. Mycopathologia. 2022 Dec;187(5-6):497-508. doi: 10.1007/s11046-022-00665-2. Epub 2022 Sep 13. Mycopathologia. 2022. PMID: 36098829 Free PMC article. - Aspergillus in the Indoor Air of Critical Areas of a Tertiary Hospital in Brazil.
Lemos MSC, Higa Junior MG, Paniago AMM, Melhem MSC, Takahashi JPF, Fava WS, Venancio FA, Martins NM, Chang MR. Lemos MSC, et al. J Fungi (Basel). 2024 Aug 1;10(8):538. doi: 10.3390/jof10080538. J Fungi (Basel). 2024. PMID: 39194864 Free PMC article. - Response of Fusarium oxysporum soil isolate to amphotericin B and fluconazole at the proteomic level.
Amatto IVDS, Simões FAO, Garzon NGDR, Marciano CL, Silva RRD, Cabral H. Amatto IVDS, et al. Braz J Microbiol. 2024 Sep;55(3):2557-2568. doi: 10.1007/s42770-024-01417-8. Epub 2024 Jul 1. Braz J Microbiol. 2024. PMID: 38954219 - Gene-specific transcriptional activation by the Aspergillus fumigatus AtrR factor requires a conserved C-terminal domain.
Ror S, Stamnes MA, Moye-Rowley WS. Ror S, et al. mSphere. 2024 Jul 30;9(7):e0042524. doi: 10.1128/msphere.00425-24. Epub 2024 Jul 8. mSphere. 2024. PMID: 38975761 Free PMC article.
References
- Brown GD, et al. Hidden killers: human fungal infections. Sci. Transl. Med. 2012;4:165rv13. - PubMed
- Schauwvlieghe AFAD, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir. Med. 2018;6:782–792. - PubMed
- Baxter CG, et al. Novel immunologic classification of aspergillosis in adult cystic fibrosis. J. Allergy Clin. Immunol. 2013;132:560–566.e10. - PubMed
- Kleinkauf et al. Risk Assessment on the Impact of Environmental Usage of Triazoles on the Development and Spread of Resistance to Medical Triazoles inAspergillusSpecies (ECDC, 2013).
Publication types
MeSH terms
Substances
Grants and funding
- 219551/Z/19/Z/WT_/Wellcome Trust/United Kingdom
- MR/N006364/2/MRC_/Medical Research Council/United Kingdom
- 097377/WT_/Wellcome Trust/United Kingdom
- DH_/Department of Health/United Kingdom
- MR/V033417/1/MRC_/Medical Research Council/United Kingdom
- MR/R015600/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MR/V037315/1/MRC_/Medical Research Council/United Kingdom
- BB/M010996/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources