Environmental DNA analysis confirms extant populations of the cryptic Irwin's turtle within its historical range - PubMed (original) (raw)
Environmental DNA analysis confirms extant populations of the cryptic Irwin's turtle within its historical range
Cecilia Villacorta-Rath et al. BMC Ecol Evol. 2022.
Abstract
Background: Approximately 50% of freshwater turtles worldwide are currently threatened by habitat loss, rural development and altered stream flows. Paradoxically, reptiles are understudied organisms, with many species lacking basic geographic distribution and abundance data. The iconic Irwin's turtle, Elseya irwini, belongs to a unique group of Australian endemic freshwater turtles capable of cloacal respiration. Water resource development, increased presence of saltwater crocodiles and its cryptic behaviour, have made sampling for Irwin's turtle in parts of its range problematic, resulting in no confirmed detections across much of its known range for > 25 years. Here, we used environmental DNA (eDNA) analysis for E. irwini detection along its historical and contemporary distribution in the Burdekin, Bowen and Broken River catchments and tributaries. Five replicate water samples were collected at 37 sites across those three river catchments. Environmental DNA was extracted using a glycogen-aided precipitation method and screened for the presence of E. irwini through an eDNA assay targeting a 127 base pair-long fragment of the NADH dehydrogenase 4 (ND4) mitochondrial gene.
Results: Elseya irwini eDNA was detected at sites within its historic distribution in the lower Burdekin River, where the species had not been formally recorded for > 25 years, indicating the species still inhabits the lower Burdekin area. We also found higher levels of E. iriwni eDNA within its contemporary distribution in the Bowen and Broken Rivers, matching the prevailing scientific view that these areas host larger populations of E. irwini.
Conclusions: This study constitutes the first scientific evidence of E. irwini presence in the lower Burdekin since the original type specimens were collected as part of its formal description, shortly after the construction of the Burdekin Falls Dam. From the higher percentage of positive detections in the upper reaches of the Broken River (Urannah Creek), we conclude that this area constitutes the core habitat area for the species. Our field protocol comprises a user-friendly, time-effective sampling method. Finally, due to safety risks associated with traditional turtle sampling methods in the Burdekin River (e.g., estuarine crocodiles) we propose eDNA sampling as the most pragmatic detection method available for E. irwini.
Keywords: Catchment-wide survey; Dam development; Elseya irwini; Monitoring; User-friendly field methods; eDNA.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
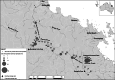
Fig. 1
Sampling sites surveyed by three different institutions during 2020 and 2021 for E. irwini eDNA detection along the Burdekin, Bowen and Broken River catchments
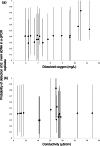
Fig. 2
Estimated probabilities of detection of E. irwini eDNA in qPCR replicates. Symbols are estimates of posterior medians, with error bars indicating 95% confidence intervals
Similar articles
- Concurrent visual encounter sampling validates eDNA selectivity and sensitivity for the endangered wood turtle (Glyptemys insculpta).
Akre TS, Parker LD, Ruther E, Maldonado JE, Lemmon L, McInerney NR. Akre TS, et al. PLoS One. 2019 Apr 24;14(4):e0215586. doi: 10.1371/journal.pone.0215586. eCollection 2019. PLoS One. 2019. PMID: 31017960 Free PMC article. - An unusual mortality event in Johnstone River snapping turtles Elseya irwini (Johnstone) in Far North Queensland, Australia.
Ariel E, Freeman AB, Elliott E, Wirth W, Mashkour N, Scott J. Ariel E, et al. Aust Vet J. 2017 Oct;95(10):355-361. doi: 10.1111/avj.12627. Aust Vet J. 2017. PMID: 28948624 - Molecular identity crisis: environmental DNA metabarcoding meets traditional taxonomy-assessing biodiversity and freshwater mussel populations (Unionidae) in Alabama.
Hauck LL, Atkinson CL, Homyack JA, Penaluna BE, Mangum C, Coble AA, Nettles J, Thornton-Frost JE, Fix MJ. Hauck LL, et al. PeerJ. 2023 Apr 3;11:e15127. doi: 10.7717/peerj.15127. eCollection 2023. PeerJ. 2023. PMID: 37033728 Free PMC article. - Salinity tolerances and use of saline environments by freshwater turtles: implications of sea level rise.
Agha M, Ennen JR, Bower DS, Nowakowski AJ, Sweat SC, Todd BD. Agha M, et al. Biol Rev Camb Philos Soc. 2018 Aug;93(3):1634-1648. doi: 10.1111/brv.12410. Epub 2018 Mar 25. Biol Rev Camb Philos Soc. 2018. PMID: 29575680 Review. - Environmental DNA/RNA for pathogen and parasite detection, surveillance, and ecology.
Bass D, Christison KW, Stentiford GD, Cook LSJ, Hartikainen H. Bass D, et al. Trends Parasitol. 2023 Apr;39(4):285-304. doi: 10.1016/j.pt.2022.12.010. Epub 2023 Feb 8. Trends Parasitol. 2023. PMID: 36759269 Review.
Cited by
- A comparative study on eDNA-based detection of Siamese bat catfish (Oreoglanis siamensis) in wet and dry conditions.
Osathanunkul M, Suwannapoom C. Osathanunkul M, et al. Sci Rep. 2024 Apr 17;14(1):8885. doi: 10.1038/s41598-024-58752-x. Sci Rep. 2024. PMID: 38632301 Free PMC article. - Exploring the relationship between environmental DNA concentration and biomass in Asian giant softshell turtle (Pelochelys cantorii).
Hong X, Wang K, Ji L, Liu X, Yu L, Wei J, Wang Y, Wei C, Li W, Zhu X. Hong X, et al. PeerJ. 2023 Oct 4;11:e16218. doi: 10.7717/peerj.16218. eCollection 2023. PeerJ. 2023. PMID: 37810767 Free PMC article.
References
- Moll D, Moll EO. The ecology, exploitation and conservation of river turtle. Oxford University Press on Demand; 2004.
- Georges A. Setting conservation priorities for Australian freshwater turtles. In: Lunney D, Ayers D, editors. Herpetology in Australia: a diverse discipline. 1993. p. 49–58.
- Rhodin AGJ, Stanford CB, van Dijk PP, Eisemberg C, Luiselli L, Mittermeier RA, et al. Global conservation status of turtles and tortoises (Order Testudines) Chelonian Conserv Biol [Internet]. 2018;17(2):135–161. doi: 10.2744/CCB-1348.1. - DOI
- Cann J, Sadlier R. Freshwater turtles of Austalia. ECO Wear & Publishing; 2017. 448 p.
- FitzGibbon SI, Franklin CE. The importance of the cloacal bursae as the primary site of aquatic respiration in the freshwater turtle, Elseya albagula. Aust Zool. 2010;35(2):276–282. doi: 10.7882/AZ.2010.016. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources