A View on Genomic Medicine Activities in Africa: Implications for Policy - PubMed (original) (raw)
doi: 10.3389/fgene.2022.769919. eCollection 2022.
Maritha J Kotze 2, Archana Bhaw-Luximon 3, Faisal M Fadlelmola 4, Yasmina J Fakim 5, Yosr Hamdi 6 7, Samar Kamal Kassim 8, Judit Kumuthini 9, Victoria Nembaware 10, Fouzia Radouani 11, Nicki Tiffin 12 13, Nicola Mulder 12 13
Affiliations
- PMID: 35571023
- PMCID: PMC9091728
- DOI: 10.3389/fgene.2022.769919
A View on Genomic Medicine Activities in Africa: Implications for Policy
C Victor Jongeneel et al. Front Genet. 2022.
Abstract
Genomics policy development involves assessing a wide range of issues extending from specimen collection and data sharing to whether and how to utilize advanced technologies in clinical practice and public health initiatives. A survey was conducted among African scientists and stakeholders with an interest in genomic medicine, seeking to evaluate: 1) Their knowledge and understanding of the field. 2) The institutional environment and infrastructure available to them. 3) The state and awareness of the field in their country. 4) Their perception of potential barriers to implementation of precision medicine. We discuss how the information gathered in the survey could instruct the policies of African institutions seeking to implement precision, and more specifically, genomic medicine approaches in their health care systems in the following areas: 1) Prioritization of infrastructures. 2) Need for translational research. 3) Information dissemination to potential users. 4) Training programs for specialized personnel. 5) Engaging political stakeholders and the public. A checklist with key requirements to assess readiness for implementation of genomic medicine programs is provided to guide the process from scientific discovery to clinical application.
Keywords: Africa; Pathology-supported genomics; capacity development; genomic medicine; infrastructure; precision medicine stakeholders; readiness checklist; translational research.
Copyright © 2022 Jongeneel, Kotze, Bhaw-Luximon, Fadlelmola, Fakim, Hamdi, Kassim, Kumuthini, Nembaware, Radouani, Tiffin and Mulder.
Conflict of interest statement
MK is a non-executive director and shareholder of Gknowmix (Pty) Ltd., that developed a database resource for research translation under the auspices of the South African Research Council. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
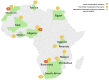
FIGURE 1
Map showing the countries from which responses were received, including the total number of respondents within the country (dark orange) and out of these, the number who are currently or planning to implement genomic medicine activities (light orange).
Similar articles
- The future of Cochrane Neonatal.
Soll RF, Ovelman C, McGuire W. Soll RF, et al. Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834 - THE FUTURE OF MEDICINE, healthcare innovation through precision medicine: policy case study of Qatar.
Qoronfleh MW, Chouchane L, Mifsud B, Al Emadi M, Ismail S. Qoronfleh MW, et al. Life Sci Soc Policy. 2020 Nov 1;16(1):12. doi: 10.1186/s40504-020-00107-1. Life Sci Soc Policy. 2020. PMID: 33129349 Free PMC article. Review. - Establishing an infrastructure to optimize the integration of genomics into research: Results from a precision health needs assessment.
Allen CG, Bouchie G, Judge DP, Coen E, English S, Norman S, Kirchoff K, Ramos PS, Hirschhorn J, Lenert L, McMahon LL. Allen CG, et al. Transl Behav Med. 2024 Jun 27;14(7):386-393. doi: 10.1093/tbm/ibae008. Transl Behav Med. 2024. PMID: 38470971 - From biobank and data silos into a data commons: convergence to support translational medicine.
Asiimwe R, Lam S, Leung S, Wang S, Wan R, Tinker A, McAlpine JN, Woo MMM, Huntsman DG, Talhouk A. Asiimwe R, et al. J Transl Med. 2021 Dec 4;19(1):493. doi: 10.1186/s12967-021-03147-z. J Transl Med. 2021. PMID: 34863191 Free PMC article. - Building a precision medicine infrastructure at a national level: The Swedish experience.
Edsjö A, Lindstrand A, Gisselsson D, Mölling P, Friedman M, Cavelier L, Johansson M, Ehrencrona H, Fagerqvist T, Strid T, Lovmar L, Jacobsson B, Johansson Å, Engstrand L, Wheelock CE, Sikora P, Wirta V, Fioretos T, Rosenquist R; Genomic Medicine Sweden (GMS). Edsjö A, et al. Camb Prism Precis Med. 2023 Feb 27;1:e15. doi: 10.1017/pcm.2023.3. eCollection 2023. Camb Prism Precis Med. 2023. PMID: 38550923 Free PMC article. Review.
Cited by
- Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC).
Pretorius E, Venter C, Laubscher GJ, Kotze MJ, Oladejo SO, Watson LR, Rajaratnam K, Watson BW, Kell DB. Pretorius E, et al. Cardiovasc Diabetol. 2022 Aug 6;21(1):148. doi: 10.1186/s12933-022-01579-5. Cardiovasc Diabetol. 2022. PMID: 35933347 Free PMC article. - Data sharing: A Long COVID perspective, challenges, and road map for the future.
Oladejo SO, Watson LR, Watson BW, Rajaratnam K, Kotze MJ, Kell DB, Pretorius E. Oladejo SO, et al. S Afr J Sci. 2023 May-Jun;119(5-6):73-80. doi: 10.17159/sajs.2023/14719. Epub 2023 May 30. S Afr J Sci. 2023. PMID: 39324014 Free PMC article. - Building genomic capacity for precision health in Africa.
Olono A, Mitesser V, Happi A, Happi C. Olono A, et al. Nat Med. 2024 Jul;30(7):1856-1864. doi: 10.1038/s41591-024-03081-9. Epub 2024 Jul 3. Nat Med. 2024. PMID: 38961224 Review. - A regionally based precision medicine implementation initiative in North Africa:The PerMediNA consortium.
Hamdi Y, Boujemaa M, Ben Aissa-Haj J, Radouani F, Khyatti M, Mighri N, Hannachi M, Ghedira K, Souiai O, Hkimi C, Kammoun MS, Mejri N, Bouaziz H, Beloufa MA, Charoute H, Barakat A, Najjar I, Taniguchi H, Pietrosemoli N; PerMediNA Consortium; Dellagi K, Abdelhak S, Boubaker MS, Chica C, Rouleau E. Hamdi Y, et al. Transl Oncol. 2024 Jun;44:101940. doi: 10.1016/j.tranon.2024.101940. Epub 2024 Mar 26. Transl Oncol. 2024. PMID: 38537326 Free PMC article. - An Assessment of the Knowledge and Perceptions of Precision Medicine (PM) in the Rwandan Healthcare Setting.
Musanabaganwa C, Ruton H, Ruhangaza D, Nsabimana N, Kayitare E, Muvunyi TZ, Semakula M, Ntirenganya F, Musoni E, Ndoli J, Hategekimana E, Nassir A, Makokha F, Uwimana A, Gasana J, Munezero PC, Uwinkindi F, Muvunyi CM, Nyirazinyoye L, Mazarati JB, Mutesa L. Musanabaganwa C, et al. J Pers Med. 2023 Dec 14;13(12):1707. doi: 10.3390/jpm13121707. J Pers Med. 2023. PMID: 38138934 Free PMC article.
References
- Mampunye L., Grant K. A., Peeters A. V., Torrorey-Sawe R., French D. J., Moremi K. E., et al. (2021). MammaPrint Risk Score Distribution in South African Breast Cancer Patients with the Pathogenic BRCA2 c.7934delG Founder Variant: Towards Application of Genomic Medicine at the Point-of-Care. Breast 56 (Suppl. 1), S17–S90. 10.1016/s0960-9776(21)00120-x - DOI
LinkOut - more resources
Full Text Sources