The paradigm shift in treatment from Covid-19 to oncology with mRNA vaccines - PubMed (original) (raw)
Review
The paradigm shift in treatment from Covid-19 to oncology with mRNA vaccines
Jiao Wei et al. Cancer Treat Rev. 2022 Jun.
Abstract
mRNA vaccines have gained popularity over the last decade as a versatile tool for developing novel therapeutics. The recent success of coronavirus disease (COVID-19) mRNA vaccine has unlocked the potential of mRNA technology as a powerful therapeutic platform. In this review, we apprise the literature on the various types of cancer vaccines, the novel platforms available for delivery of the vaccines, the recent progress in the RNA-based therapies and the evolving role of mRNA vaccines for various cancer indications, along with a future strategy to treat the patients. Literature reveals that despite multifaceted challenges in the development of mRNA vaccines, the promising and durable efficacy of the RNA in pre-clinical and clinical studies deserves consideration. The introduction of mRNA-transfected DC vaccine is an approach that has gained interest for cancer vaccine development due to its ability to circumvent the necessity of DC isolation, ex vivo cultivation and re-infusion. The selection of appropriate antigen of interest remains one of the major challenges for cancer vaccine development. The rapid development and large-scale production of mRNA platform has enabled for the development of both personalized vaccines (mRNA 4157, mRNA 4650 and RO7198457) and tetravalent vaccines (BNT111 and mRNA-5671). In addition, mRNA vaccines combined with checkpoint modulators and other novel medications that reverse immunosuppression show promise, however further research is needed to discover which combinations are most successful and the best dosing schedule for each component. Each delivery route (intradermal, subcutaneous, intra tumoral, intranodal, intranasal, intravenous) has its own set of challenges to overcome, and these challenges will decide the best delivery method. In other words, while developing a vaccine design, the underlying motivation should be a reasonable combination of delivery route and format. Exploring various administration routes and delivery route systems has boosted the development of mRNA vaccines.
Keywords: Cancer vaccine; Covid-19; Oncology; Optimization; mRNA.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Fig. 1
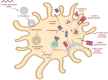
The commonly available platforms and mechanisms for cancer vaccine development. (a) Whole cell-based vaccines (an autologous tumor cell vaccine using a patient’s own cancer cells is injected as vaccine). (b) Viral vector-based vaccines (the genome of viral particles is modified to contain one or more genes encoding for the antigens of interest). (c) Dendritic cell-based vaccines (the dendritic cells efficiently capture the antigens, internalize, and process into peptides that are then presented in the context of MHC I and II molecules. These complexes are later recognized by the T-cell receptor (TCR) of CD8+ and CD4 + T cells) (d) DNA based vaccines (DNA plasmids are designed to deliver genes encoding TAs, eliciting or augmenting the adaptive immune response towards TA-bearing tumor cells. It induces the innate immune response, stimulates several DNA-sensing pathways in the cytosol of transfected cells due to the presence of CpG motifs and the double stranded structure itself) (e) Peptide-based vaccines (the peptides bind with the restricted MHC molecule expressed in APC. The peptide/MHC complex is then transported to the cell surface after intracellular processing and later recognized by the TCR on the surface of T cells, leading to activation of T lymphocytes) (f) RNA based vaccines (conventional non-replicating mRNA consists of 5 structural elements such as cap structures, a 5′ untranslated region (5′-UTR), an open reading frame encoding antigens of interest, a 3′-UTR; and an adenine repeating nucleotide sequence that forms a polyadenine (poly(A) tail. The non-replicating mRNA encodes antigen of interest, while self-amplifying mRNA encodes antigen of interest and a replication machinery, a self-replicating single-stranded RNA virus).
Fig. 2
Mechanism of action of mRNA vaccines. 1. In a cell-free system, mRNA is in vitro transcribed (IVT) from a DNA template. 2. IVT mRNA is then transfected into dendritic cells (DCs) by the process of (3) endocytosis. 4. Endosomal escape allows entrapped mRNA to be released into the cytoplasm. 5. The mRNA is translated into antigenic proteins using the ribosome translational mechanism. After post-translational modification, the translated antigenic protein is ready to act in the cell where it was produced. 6. The protein gets secreted by the host cell. 7. Antigen proteins are digested in the cytoplasm by the proteasome and transferred to the endoplasmic reticulum, where they are loaded onto MHC class I molecules (MHC I). 8. MHC I-peptide epitope complexes with loaded MHC I-peptide epitopes produced, resulting in induction. 9. Exogenous proteins are taken up DCs. 10. They are degraded in endosomes and delivered via the MHC II pathway. Furthermore, to obtain cognate T-cell help in antigen-presenting cells, the protein should be routed through the MHC II pathway. 11. The generated antigenic peptide epitopes are subsequently loaded onto MHC II molecules.
Fig. 3
Key elements that affect mRNA vaccine stability and translation efficacy. LNP – lipid nanoparticles, RNA – ribonucleic acid, UTR – untranslated region.
Similar articles
- mRNA vaccines: a new era in vaccine development.
Chandra S, Wilson JC, Good D, Wei MQ. Chandra S, et al. Oncol Res. 2024 Sep 18;32(10):1543-1564. doi: 10.32604/or.2024.043987. eCollection 2024. Oncol Res. 2024. PMID: 39308511 Free PMC article. Review. - The Rapid Development and Early Success of Covid 19 Vaccines Have Raised Hopes for Accelerating the Cancer Treatment Mechanism.
Amanpour S. Amanpour S. Arch Razi Inst. 2021 Mar;76(1):1-6. doi: 10.22092/ari.2021.353761.1612. Epub 2021 Mar 1. Arch Razi Inst. 2021. PMID: 33818952 Free PMC article. - Prospects and Challenges in Developing mRNA Vaccines for Infectious Diseases and Oncogenic Viruses.
Kutikuppala LVS, Kourampi I, Kanagala RSD, Bhattacharjee P, Boppana SH. Kutikuppala LVS, et al. Med Sci (Basel). 2024 May 22;12(2):28. doi: 10.3390/medsci12020028. Med Sci (Basel). 2024. PMID: 38804384 Free PMC article. Review. - [Vaccine development based on RNA technology platforms].
Guo X, Li J, Wang HM, Qiu J, Li Z, Huang F, Li J, Sun XD. Guo X, et al. Zhonghua Yu Fang Yi Xue Za Zhi. 2024 Aug 6;58(8):1263-1277. doi: 10.3760/cma.j.cn112150-20230831-00147. Zhonghua Yu Fang Yi Xue Za Zhi. 2024. PMID: 39142899 Review. Chinese. - mRNA vaccine for cancer immunotherapy.
Miao L, Zhang Y, Huang L. Miao L, et al. Mol Cancer. 2021 Feb 25;20(1):41. doi: 10.1186/s12943-021-01335-5. Mol Cancer. 2021. PMID: 33632261 Free PMC article. Review.
Cited by
- The transformative potential of mRNA vaccines for glioblastoma and human cancer: technological advances and translation to clinical trials.
Tapescu I, Madsen PJ, Lowenstein PR, Castro MG, Bagley SJ, Fan Y, Brem S. Tapescu I, et al. Front Oncol. 2024 Sep 27;14:1454370. doi: 10.3389/fonc.2024.1454370. eCollection 2024. Front Oncol. 2024. PMID: 39399167 Free PMC article. Review. - The progress of tumor vaccines clinical trials in non-small cell lung cancer.
Wang X, Niu Y, Bian F. Wang X, et al. Clin Transl Oncol. 2024 Aug 23. doi: 10.1007/s12094-024-03678-z. Online ahead of print. Clin Transl Oncol. 2024. PMID: 39179939 Review. - From structural design to delivery: mRNA therapeutics for cancer immunotherapy.
Zhou F, Huang L, Li S, Yang W, Chen F, Cai Z, Liu X, Xu W, Lehto VP, Lächelt U, Huang R, Shi Y, Lammers T, Tao W, Xu ZP, Wagner E, Xu Z, Yu H. Zhou F, et al. Exploration (Beijing). 2023 Nov 17;4(2):20210146. doi: 10.1002/EXP.20210146. eCollection 2024 Apr. Exploration (Beijing). 2023. PMID: 38855617 Free PMC article. Review. - KRAS: Biology, Inhibition, and Mechanisms of Inhibitor Resistance.
Ash LJ, Busia-Bourdain O, Okpattah D, Kamel A, Liberchuk A, Wolfe AL. Ash LJ, et al. Curr Oncol. 2024 Apr 3;31(4):2024-2046. doi: 10.3390/curroncol31040150. Curr Oncol. 2024. PMID: 38668053 Free PMC article. Review. - Advances in Therapeutic Cancer Vaccines, Their Obstacles, and Prospects Toward Tumor Immunotherapy.
Eskandari A, Leow TC, Rahman MBA, Oslan SN. Eskandari A, et al. Mol Biotechnol. 2024 Apr 16. doi: 10.1007/s12033-024-01144-3. Online ahead of print. Mol Biotechnol. 2024. PMID: 38625508 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical