Intestinal fatty acid binding protein is a disease biomarker in paediatric coeliac disease and Crohn's disease - PubMed (original) (raw)
Intestinal fatty acid binding protein is a disease biomarker in paediatric coeliac disease and Crohn's disease
Michael Logan et al. BMC Gastroenterol. 2022.
Abstract
Background: There is a clinical need to develop biomarkers of small bowel damage in coeliac disease and Crohn's disease. This study evaluated intestinal fatty acid binding protein (iFABP), a potential biomarker of small bowel damage, in children with coeliac disease and Crohn's disease.
Methods: The concentration iFABP was measured in plasma and urine of children with ulcerative colitis, coeliac disease, and Crohn's disease at diagnosis and from the latter two groups after treatment with gluten free diet (GFD) or exclusive enteral nutrition (EEN), respectively. Healthy children (Controls) were also recruited.
Results: 138 children were recruited. Plasma but not urinary iFABP was higher in patients with newly diagnosed coeliac disease than Controls (median [Q1, Q3] coeliac disease: 2104 pg/mL 1493, 2457] vs Controls: 938 pg/mL [616, 1140], p = 0.001). Plasma or urinary iFABP did not differ between patients with coeliac on GFD and Controls. Baseline iFABP in plasma decreased by 6 months on GFD (6mo GFD: 1238 pg/mL [952, 1618], p = 0.045). By 12 months this effect was lost, at which point 25% of patients with coeliac disease had detectable gluten in faeces, whilst tissue transglutaminase IgA antibodies (TGA) continued to decrease. At diagnosis, patients with Crohn's disease had higher plasma iFABP levels than Controls (EEN Start: 1339 pg/mL [895, 1969] vs Controls: 938 pg/mL [616, 1140], p = 0.008). iFABP did not differ according to Crohn's disease phenotype. Induction treatment with EEN tended to decrease (p = 0.072) iFABP in plasma which was no longer different to Controls (EEN End: 1114 pg/mL [689, 1400] vs Controls: 938 pg/mL [616, 1140], p = 0.164). Plasma or urinary iFABP did not differ in patients with ulcerative colitis from Controls (plasma iFABP, ulcerative colitis: 1309 pg/mL [1005, 1458] vs Controls: 938 pg/mL [616, 1140], p = 0.301; urinary iFABP ulcerative colitis: 38 pg/mg [29, 81] vs Controls: 53 pg/mg [27, 109], p = 0.605).
Conclusions: Plasma, but not urinary iFABP is a candidate biomarker with better fidelity in monitoring compliance during GFD than TGA. The role of plasma iFABP in Crohn's disease is promising but warrants further investigation.
Trial registration: Clinical Trials.gov, NCT02341248. Registered on 19/01/2015.
Keywords: Biomarkers; Coeliac disease; Crohn’s disease; Inflammatory bowel disease; Paediatric; Ulcerative colitis.
© 2022. The Author(s).
Conflict of interest statement
KG received research grants, speakers’ fees and had conference attendance paid by Nutricia, Nestle, Abbott, Baxter and Dr Falk. UZI is supported by NERC NE/L011956/1. RH had received speakers/consultancy fees or conference support from Nutricia and 4D Pharma. RKR has received speaker’s fees, travel support, and/or participated in medical board meetings with Nestle, MSD Immunology, AbbVie, Dr Falk, Takeda, Napp, Mead Johnson, Nutricia & 4D Pharma.
Figures
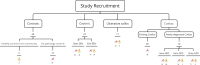
Fig. 1
Schematic representation of study recruitment with sample type collection in each study group. Abbreviations mo: month, GFD: gluten-free diet
Fig. 2
Boxplots A plasma, B urinary iFABP concentrations in Controls and patients with Coeliac during GFD; C plasma, D urinary iFABP concentration in Controls and patients with Colitis and Crohn’s during exclusive enteral nutrition. Coeliac: coeliac disease, ND: newly diagnosed, Crohn’s: Crohn’s disease, Controls: healthy control, mo: month, GFD: gluten-free diet, EEN: exclusive enteral nutrition. Urinary iFABP measurements are expressed after urinary creatinine correction, a: p < 0.05 compared with Controls; b: p < 0.05 compared with ND Coeliac
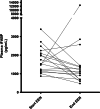
Fig. 3
Individual value plot of pairwise plasma iFABP levels in patients with Crohn's during treatment with exclusive enteral nutrition
Similar articles
- Dietary triggers of gut inflammation following exclusive enteral nutrition in children with Crohn's disease: a pilot study.
Gkikas K, Logan M, Nichols B, Ijaz UZ, Clark CM, Svolos V, Gervais L, Duncan H, Garrick V, Curtis L, Buchanan E, Cardigan T, Armstrong L, Delahunty C, Flynn DM, Barclay AR, Tayler R, Milling S, Hansen R, Russell RK, Gerasimidis K. Gkikas K, et al. BMC Gastroenterol. 2021 Dec 3;21(1):454. doi: 10.1186/s12876-021-02029-4. BMC Gastroenterol. 2021. PMID: 34861829 Free PMC article. - Risk of schizophrenia in people with coeliac disease, ulcerative colitis and Crohn's disease: a general population-based study.
West J, Logan RF, Hubbard RB, Card TR. West J, et al. Aliment Pharmacol Ther. 2006 Jan 1;23(1):71-4. doi: 10.1111/j.1365-2036.2006.02720.x. Aliment Pharmacol Ther. 2006. PMID: 16393282 - Plasma levels of endothelin-1 in patients with Crohn's disease and ulcerative colitis.
Letizia C, Boirivant M, De Toma G, Cerci S, Subioli S, Scuro L, Ferrari P, Pallone F. Letizia C, et al. Ital J Gastroenterol Hepatol. 1998 Jun;30(3):266-9. Ital J Gastroenterol Hepatol. 1998. PMID: 9759593 - [Coexistence of coeliac disease and inflammatory bowel disease in children].
Krawiec P, Pawłowska-Kamieniak A, Pac-Kożuchowska E, Mroczkowska-Juchkiewcz A, Kominek K. Krawiec P, et al. Pol Merkur Lekarski. 2016 Jan;40(235):53-5. Pol Merkur Lekarski. 2016. PMID: 26891438 Review. Polish. - Exclusive enteral nutrition in Crohn's disease: Evidence and practicalities.
Ashton JJ, Gavin J, Beattie RM. Ashton JJ, et al. Clin Nutr. 2019 Feb;38(1):80-89. doi: 10.1016/j.clnu.2018.01.020. Epub 2018 Feb 15. Clin Nutr. 2019. PMID: 29398336 Review.
Cited by
- Urinary chitinase 3-like 1 and intestinal fatty-acid binding proteins are not elevated in children with inflammatory bowel disease.
Ho SSC, Keenan JI, Day AS. Ho SSC, et al. Intest Res. 2022 Oct;20(4):509-513. doi: 10.5217/ir.2022.00064. Epub 2022 Jul 22. Intest Res. 2022. PMID: 35860847 Free PMC article. No abstract available. - Intestinal Fatty Acid Binding Protein (I-FABP) as a Prognostic Marker in Critically Ill COVID-19 Patients.
Tyszko M, Lipińska-Gediga M, Lemańska-Perek A, Kobylińska K, Gozdzik W, Adamik B. Tyszko M, et al. Pathogens. 2022 Dec 13;11(12):1526. doi: 10.3390/pathogens11121526. Pathogens. 2022. PMID: 36558860 Free PMC article. - Markers of Intestinal Permeability and Inflammation in Enterally Fed Children with Cerebral Palsy.
Mickiewicz-Góra D, Sznurkowska K, Skonieczna-Żydecka K, Drozd A, Borkowska A, Zagierski M, Troch J, Szlagatys-Sidorkiewicz A. Mickiewicz-Góra D, et al. Nutrients. 2024 Jul 27;16(15):2447. doi: 10.3390/nu16152447. Nutrients. 2024. PMID: 39125328 Free PMC article. - Intestinal fatty acid binding protein is associated with coronary artery disease in long-term type 1 diabetes-the Dialong study.
Narum M, Seljeflot I, Bratseth V, Berg TJ, Sveen KA. Narum M, et al. Cardiovasc Diabetol. 2024 Nov 19;23(1):419. doi: 10.1186/s12933-024-02509-3. Cardiovasc Diabetol. 2024. PMID: 39563343 Free PMC article. - Gut Microbiota and Biomarkers of Intestinal Barrier Damage in Cirrhosis.
Efremova I, Maslennikov R, Medvedev O, Kudryavtseva A, Avdeeva A, Krasnov G, Romanikhin F, Diatroptov M, Fedorova M, Poluektova E, Levshina A, Ivashkin V. Efremova I, et al. Microorganisms. 2024 Feb 25;12(3):463. doi: 10.3390/microorganisms12030463. Microorganisms. 2024. PMID: 38543514 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous