Performance of Antigen Detection Tests for SARS-CoV-2: A Systematic Review and Meta-Analysis - PubMed (original) (raw)
Review
Performance of Antigen Detection Tests for SARS-CoV-2: A Systematic Review and Meta-Analysis
Anastasia Tapari et al. Diagnostics (Basel). 2022.
Abstract
Coronavirus disease 2019 (COVID-19) initiated global health care challenges such as the necessity for new diagnostic tests. Diagnosis by real-time PCR remains the gold-standard method, yet economical and technical issues prohibit its use in points of care (POC) or for repetitive tests in populations. A lot of effort has been exerted in developing, using, and validating antigen-based tests (ATs). Since individual studies focus on few methodological aspects of ATs, a comparison of different tests is needed. Herein, we perform a systematic review and meta-analysis of data from articles in PubMed, medRxiv and bioRxiv. The bivariate method for meta-analysis of diagnostic tests pooling sensitivities and specificities was used. Most of the AT types for SARS-CoV-2 were lateral flow immunoassays (LFIA), fluorescence immunoassays (FIA), and chemiluminescence enzyme immunoassays (CLEIA). We identified 235 articles containing data from 220,049 individuals. All ATs using nasopharyngeal samples show better performance than those with throat saliva (72% compared to 40%). Moreover, the rapid methods LFIA and FIA show about 10% lower sensitivity compared to the laboratory-based CLEIA method (72% compared to 82%). In addition, rapid ATs show higher sensitivity in symptomatic patients compared to asymptomatic patients, suggesting that viral load is a crucial parameter for ATs performed in POCs. Finally, all methods perform with very high specificity, reaching around 99%. LFIA tests, though with moderate sensitivity, appear as the most attractive method for use in POCs and for performing seroprevalence studies.
Keywords: COVID-19; SARS-CoV-2; antigen test; diagnostic performance; meta-analysis; sensitivity; specificity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
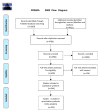
Figure 1
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
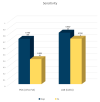
Figure 2
Performance of POC (LFIA and FIA) and laboratory (CLEIA) antigen-based methods in terms of sensitivity. All included assays in the meta-analysis use samples with Ct < 40 and test cumulatively both the nucleocapsid and Spike antigen. Numbers above the bars depict sensitivity values/number of studies included in each meta-analysis.
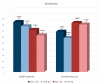
Figure 3
Performance of LFIA and FIA methods (N antigen-based) in terms of sensitivity on NSP samples in symptomatic vs. asymptomatic persons. Included assays in the meta-analysis are performed with positive samples for either Ct < 30 or Ct < 40. Numbers above the bars depict sensitivity values/number of studies included in each meta-analysis.
Similar articles
- Antibody Tests in Detecting SARS-CoV-2 Infection: A Meta-Analysis.
Kontou PI, Braliou GG, Dimou NL, Nikolopoulos G, Bagos PG. Kontou PI, et al. Diagnostics (Basel). 2020 May 19;10(5):319. doi: 10.3390/diagnostics10050319. Diagnostics (Basel). 2020. PMID: 32438677 Free PMC article. - Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.
Dinnes J, Sharma P, Berhane S, van Wyk SS, Nyaaba N, Domen J, Taylor M, Cunningham J, Davenport C, Dittrich S, Emperador D, Hooft L, Leeflang MM, McInnes MD, Spijker R, Verbakel JY, Takwoingi Y, Taylor-Phillips S, Van den Bruel A, Deeks JJ; Cochrane COVID-19 Diagnostic Test Accuracy Group. Dinnes J, et al. Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article. Review. - Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: Two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech).
Nicol T, Lefeuvre C, Serri O, Pivert A, Joubaud F, Dubée V, Kouatchet A, Ducancelle A, Lunel-Fabiani F, Le Guillou-Guillemette H. Nicol T, et al. J Clin Virol. 2020 Aug;129:104511. doi: 10.1016/j.jcv.2020.104511. Epub 2020 Jun 15. J Clin Virol. 2020. PMID: 32593133 Free PMC article. - Seropositivity rate and diagnostic accuracy of serological tests in 2019-nCoV cases: a pooled analysis of individual studies.
Guo CC, Mi JQ, Nie H. Guo CC, et al. Eur Rev Med Pharmacol Sci. 2020 Oct;24(19):10208-10218. doi: 10.26355/eurrev_202010_23243. Eur Rev Med Pharmacol Sci. 2020. PMID: 33090430 Review. - Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis.
Lisboa Bastos M, Tavaziva G, Abidi SK, Campbell JR, Haraoui LP, Johnston JC, Lan Z, Law S, MacLean E, Trajman A, Menzies D, Benedetti A, Ahmad Khan F. Lisboa Bastos M, et al. BMJ. 2020 Jul 1;370:m2516. doi: 10.1136/bmj.m2516. BMJ. 2020. PMID: 32611558 Free PMC article.
Cited by
- SARS-CoV-2 antigen detection by saliva; an alternative to nasopharyngeal specimen: A cross-sectional study.
Jubair M, Rahman S, Begum MN, Talha M, Musarrat R, Rahman AKMS, Uddin MS, Wen A, Ning Y, Nahar K, Rahman MZ, Rahman M. Jubair M, et al. Health Sci Rep. 2023 May 18;6(5):e1275. doi: 10.1002/hsr2.1275. eCollection 2023 May. Health Sci Rep. 2023. PMID: 37216057 Free PMC article. - Salivary biomarkers as key to monitor personalized oral healthcare and precision dentistry: A scoping review.
Paqué PN, Hjerppe J, Zuercher AN, Jung RE, Joda T. Paqué PN, et al. Front Oral Health. 2022 Sep 22;3:1003679. doi: 10.3389/froh.2022.1003679. eCollection 2022. Front Oral Health. 2022. PMID: 36338569 Free PMC article. - Infection of the oral cavity with SARS-CoV-2 variants: Scope of salivary diagnostics.
Iyer P, Chino T, Ojcius DM. Iyer P, et al. Front Oral Health. 2022 Oct 31;3:1001790. doi: 10.3389/froh.2022.1001790. eCollection 2022. Front Oral Health. 2022. PMID: 36389278 Free PMC article. Review. - Economic Evaluation of Wastewater Surveillance Combined with Clinical COVID-19 Screening Tests, Japan.
Yoo BK, Iwamoto R, Chung U, Sasaki T, Kitajima M. Yoo BK, et al. Emerg Infect Dis. 2023 Aug;29(8):1608-1617. doi: 10.3201/eid2908.221775. Emerg Infect Dis. 2023. PMID: 37486197 Free PMC article. - Rapid antigen-based and rapid molecular tests for the detection of SARS-CoV-2: a rapid review with network meta-analysis of diagnostic test accuracy studies.
Veroniki AA, Tricco AC, Watt J, Tsokani S, Khan PA, Soobiah C, Negm A, Doherty-Kirby A, Taylor P, Lunny C, McGowan J, Little J, Mallon P, Moher D, Wong S, Dinnes J, Takwoingi Y, Saxinger L, Chan A, Isaranuwatchai W, Lander B, Meyers A, Poliquin G, Straus SE. Veroniki AA, et al. BMC Med. 2023 Mar 29;21(1):110. doi: 10.1186/s12916-023-02810-0. BMC Med. 2023. PMID: 36978074 Free PMC article. Review.
References
- IHME Institute for Health Metrics and Evaluation—COVID-19 Results Briefing. [(accessed on 30 March 2022)]. Available online: https://www.healthdata.org/COVID/updates.
- Hyams C., Marlow R., Maseko Z., King J., Ward L., Fox K., Heath R., Tuner A., Friedrich Z., Morrison L., et al. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: A test-negative, case-control study. Lancet Infect. Dis. 2021;21:1539–1548. doi: 10.1016/S1473-3099(21)00330-3. - DOI - PMC - PubMed
Publication types
Grants and funding
This research received no external funding.
LinkOut - more resources
Full Text Sources
Miscellaneous