Efficacy of polyherbal formulations for prevention of COVID-19 infection in high-risk subjects: A randomized open-label controlled clinical trial - PubMed (original) (raw)
Randomized Controlled Trial
. 2022 Sep;36(9):3632-3643.
doi: 10.1002/ptr.7531. Epub 2022 Jul 5.
Ayan Kumar Das 2, Sumeera Banday 3, Naushad Ali Rana 4, Mohini Arora 1, Sonal Jain 5, Farzana Islam 6, Shashank Agarwal 4, Varun Kashyap 6, Santosh Joshi 4, Asad Mueed 4, Mridu Dudeja 2
Affiliations
- PMID: 35791089
- PMCID: PMC9350217
- DOI: 10.1002/ptr.7531
Randomized Controlled Trial
Efficacy of polyherbal formulations for prevention of COVID-19 infection in high-risk subjects: A randomized open-label controlled clinical trial
Kailash Chandra et al. Phytother Res. 2022 Sep.
Abstract
COVID-19 is arguably the biggest health crisis the world has faced in the 21st century. Therefore, two of the polyherbal formulations, Infuza and Kulzam were assessed for the prevention of COVID-19 infection as a repurposed medication. Four hundred seven high-risk subjects were recruited in the present open-label randomized controlled clinical trial for eligibility. After assessment for eligibility, remaining 251 subjects were randomized to the test and control groups. Further, 52 high-risk subjects in Infuza, 51 in Kulzam, 51 in Infuza & Kulzam and 53 in control group completed the 14 days of intervention/assessment. The phenotyping of lymphocytes at baseline (0 day) and after 14 days of treatment was carried out by flow cytometry assays. A total of 15.09% high-risk subjects in control group turned positive as compared to only 7.69% in Infuza, 3.92% in Kulzam and 1.96% in Infuza & Kulzam groups. The rate of conversion to COVID-19 infection in Infuza & Kulzam group was minimal and statistically significant as compared to control group (p0.017). No significant changes in phenotype of lymphocytes (T, B, NK cells), absolute lymphocyte count and cytokine levels were found in study groups. However, there was a decreasing trend of hs-CRP level in high-risk subjects after intervention of polyherbal formulations for 14 days. The combination of Infuza and Kulzam may synergistically prevent COVID-19 infection in high-risk subjects of COVID-19.
Keywords: 2019 novel coronavirus disease; Infuza; Kulzam; clinical trial; inflammation; phytochemicals; phytotherapy.
© 2022 John Wiley & Sons Ltd.
Conflict of interest statement
We confirm that there is no conflict of interest associated with this publication. The medications were provided by M/S Hamdard Laboratories (Medicine Division), India. Clinical trial was conducted at Hamdard Institute of Medical Sciences and Research and associated HAHC Hospital, New Delhi. M/S Hamdard Laboratories is not involved in any aspect of clinical trial reported in the study.
Figures
FIGURE 1
(A) HP‐TLC analysis of Infuza. The plate was derivatized with 10% methanolic sulfuric acid. Brown colour band of Glycyrrhizic acid was detected in sample and standard at Rf value of 0.15. (B) Gas Chromatogram of Kulzam polyherbal formulation. The peak of camphor, menthol and thymol is present in gas chromatogram of Kulzam polyherbal formulation at Rt 17.11, 18.79, 20.65, respectively and further it is matched with the chromatogram of standard solutions; Camphor (C), Menthol (D) and Thymol (D)
FIGURE 2
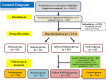
CONSORT diagram of the study
FIGURE 3
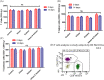
Level of T‐cells (CD3+), T‐helper (CD3+, CD4+) and T‐cytotoxic cells (CD3+, CD8+) of high‐risk subjects in study groups and alteration after administration of test drugs. (A): mean level of T‐cells (CD3+), (B): T‐helper cells (CD3+, CD4+), (C) T‐cytotoxic cells (CD3+, CD8+) at baseline (0 day) and after intervention of test drugs for 14 days. (D); Flow cytogram (BD FACS Diva 8.0.1) of T‐helper and cytotoxic cells in study subject. Data are expressed as mean ± SEM; 0 day data; collected at the time of recruitment without intervention (baseline). 14 days of data collected after giving intervention; _p_‐value is calculated using paired “_t_” test between 0 day and 14 days data. Further, 14 days data of each group were compared each other using post hoc “Tukey” multiple comparison test. The difference among all these groups is not statistically significant (p > .05: not significant)
FIGURE 4
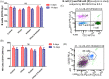
Levels of B cells (CD19+) and Natural Killer (NK) cells (CD16+, CD56+) in high‐risk subjects and alteration after administration of test drugs. (A): mean level of B cells (CD19+); (B) NK cells (CD16+, CD56+) at baseline (0 day) and after intervention of test drugs for 14 days. (C) & (D); Flow cytogram (BD FACS Diva 8.0.1) of B and NK cells in study subject. Data are expressed as mean ± SEM; 0 day data; collected at the time of recruitment without intervention (baseline). 14 days of data collected after giving intervention; _p_‐value is calculated using paired “_t_” test between 0 day and 14 days data. Further, 14 days data of each group were compared each other using post hoc “Tukey” multiple comparison test. The difference among all these groups is not statistically significant (p > .05: not significant)
Similar articles
- Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.
Emadi A, Chua JV, Talwani R, Bentzen SM, Baddley J. Emadi A, et al. Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article. - Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.
Dioh W, Chabane M, Tourette C, Azbekyan A, Morelot-Panzini C, Hajjar LA, Lins M, Nair GB, Whitehouse T, Mariani J, Latil M, Camelo S, Lafont R, Dilda PJ, Veillet S, Agus S. Dioh W, et al. Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article. - Management of Renin-Angiotensin-Aldosterone System blockade in patients admitted to hospital with confirmed coronavirus disease (COVID-19) infection (The McGill RAAS-COVID- 19): A structured summary of a study protocol for a randomized controlled trial.
Aflaki M, Flannery A, Ferreira JP, Cheng MP, Kronfli N, Marelli A, Zannad F, Brophy J, Afillalo J, Huynh T, Giannetti N, Bessissow A, Ezekowitz JA, Lopes RD, Ambrosy AP, Craig M, Sharma A. Aflaki M, et al. Trials. 2021 Feb 5;22(1):115. doi: 10.1186/s13063-021-05080-4. Trials. 2021. PMID: 33546734 Free PMC article. - Recombinant human C1 esterase inhibitor (conestat alfa) in the prevention of severe SARS-CoV-2 infection in hospitalized patients with COVID-19: A structured summary of a study protocol for a randomized, parallel-group, open-label, multi-center pilot trial (PROTECT-COVID-19).
Urwyler P, Charitos P, Moser S, Heijnen IAFM, Trendelenburg M, Thoma R, Sumer J, Camacho-Ortiz A, Bacci MR, Huber LC, Stüssi-Helbling M, Albrich WC, Sendi P, Osthoff M. Urwyler P, et al. Trials. 2021 Jan 4;22(1):1. doi: 10.1186/s13063-020-04976-x. Trials. 2021. PMID: 33397449 Free PMC article.
Cited by
- The role of TLR4/NF-kB signaling axis in pneumonia: from molecular mechanisms to regulation by phytochemicals.
Yin J, Huang J, Zhou P, Li L, Zheng Q, Fu H. Yin J, et al. Naunyn Schmiedebergs Arch Pharmacol. 2025 May 16. doi: 10.1007/s00210-025-04130-x. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40377682 Review. - Rosmarinus officinalis and Skin: Antioxidant Activity and Possible Therapeutical Role in Cutaneous Diseases.
Li Pomi F, Papa V, Borgia F, Vaccaro M, Allegra A, Cicero N, Gangemi S. Li Pomi F, et al. Antioxidants (Basel). 2023 Mar 9;12(3):680. doi: 10.3390/antiox12030680. Antioxidants (Basel). 2023. PMID: 36978928 Free PMC article. Review.
References
- Alabboud, M. , & Javadmanesh, A. (2020). In silico study of various antiviral drugs, vitamins, and natural substances as potential binding compounds with SARS‐CoV‐2 main protease. DYSONA ‐ Life Science, 1(2), 44–63. 10.30493/DLS.2020.225404 - DOI
- Andrade, B. S. , de Rangel, F. S. , Santos, N. O. , dos Freitas, A. S. , de Soares, W. R. A. , Siqueira, S. , … de Azevedo, V. A. C. (2020). Repurposing approved drugs for guiding COVID‐19 prophylaxis: A systematic review. Frontiers in Pharmacology, 11, 1–7. 10.3389/fphar.2020.590598 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous