Cell Autophagy in NASH and NASH-Related Hepatocellular Carcinoma - PubMed (original) (raw)
Review
Cell Autophagy in NASH and NASH-Related Hepatocellular Carcinoma
Utibe-Abasi S Udoh et al. Int J Mol Sci. 2022.
Abstract
Autophagy, a cellular self-digestion process, involves the degradation of targeted cell components such as damaged organelles, unfolded proteins, and intracellular pathogens by lysosomes. It is a major quality control system of the cell and plays an important role in cell differentiation, survival, development, and homeostasis. Alterations in the cell autophagic machinery have been implicated in several disease conditions, including neurodegeneration, autoimmunity, cancer, infection, inflammatory diseases, and aging. In non-alcoholic fatty liver disease, including its inflammatory form, non-alcoholic steatohepatitis (NASH), a decrease in cell autophagic activity, has been implicated in the initial development and progression of steatosis to NASH and hepatocellular carcinoma (HCC). We present an overview of autophagy as it occurs in mammalian cells with an insight into the emerging understanding of the role of autophagy in NASH and NASH-related HCC.
Keywords: NASH; NASH-related HCC; cell autophagy.
Conflict of interest statement
The authors have no conflict of interest to declare with respect to authorship and publication of this article.
Figures
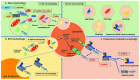
Figure 1
Autophagic pathways in mammalian cells. There are three major autophagic processes identified in most mammalian cells. (A) Macro-autophagy: in MA, cytosolic substrates determined to be transported are first packaged into autophagosomes, which are double-membrane vesicles formed through conjugation of autophagy-related proteins (i.e., Atg5, Atg12, Atg16) and autophagy-related protein LC3 with lipid PE. The formation of these autophagosomes is initiated by the phosphorylation of lipids in the membrane of organelles such as the endoplasmic reticulum, mitochondria, and Golgi apparatus, triggered by a kinase complex regulated by Beclin-1. Autophagosomes are targeted to lysosomes, and, after fusion of both vesicles, the cargo is delivered to the lysosomes for complete degradation. (B) Micro-autophagy: the in-bulk mA pathway allows cytosolic proteins and organelles to be degraded in bulk through invaginations at the lysosomal membrane. Through endosomal mA, cytosolic proteins can be selectively identified by Hsc70 to be transported to late endosomes using the KFERQ-like motif on the target proteins, leading to their internalization and degradation in the lysosomes. (C) Chaperone mediated autophagy: proteins in the cytosol with a KFERQ-like motif in their sequence can be identified by a molecular chaperone, Hsc70, and brought to the lysosomal membrane for translocation across the LAMP-2A multimeric complex. Lysosomal Hsc70 assist the translocation of the substrate protein, which is then degraded once inside the lysosomes (Adapted and modified from Scrivo et al. 2018) [20].
Similar articles
- Autophagy and non-alcoholic fatty liver disease.
Lavallard VJ, Gual P. Lavallard VJ, et al. Biomed Res Int. 2014;2014:120179. doi: 10.1155/2014/120179. Epub 2014 Sep 10. Biomed Res Int. 2014. PMID: 25295245 Free PMC article. Review. - Autophagy in nonalcoholic steatohepatitis.
Amir M, Czaja MJ. Amir M, et al. Expert Rev Gastroenterol Hepatol. 2011 Apr;5(2):159-66. doi: 10.1586/egh.11.4. Expert Rev Gastroenterol Hepatol. 2011. PMID: 21476911 Free PMC article. Review. - The Emerging Role of Extracellular Vesicles and Autophagy Machinery in NASH-Future Horizons in NASH Management.
Trifylli EM, Kriebardis AG, Koustas E, Papadopoulos N, Deutsch M, Aloizos G, Fortis SP, Papageorgiou EG, Tsagarakis A, Manolakopoulos S. Trifylli EM, et al. Int J Mol Sci. 2022 Oct 12;23(20):12185. doi: 10.3390/ijms232012185. Int J Mol Sci. 2022. PMID: 36293042 Free PMC article. Review. - The different expression of tumor suppressors, RASSF1A, RUNX3, and GSTP1, in patients with alcoholic steatohepatitis (ASH) vs non-alcoholic steatohepatitis (NASH).
Jia Y, Ji P, French B, Tillman B, French SW. Jia Y, et al. Exp Mol Pathol. 2019 Jun;108:156-163. doi: 10.1016/j.yexmp.2019.04.002. Epub 2019 Apr 3. Exp Mol Pathol. 2019. PMID: 30951700 - Molecular Pathogenesis of Nonalcoholic Steatohepatitis- (NASH-) Related Hepatocellular Carcinoma.
Kutlu O, Kaleli HN, Ozer E. Kutlu O, et al. Can J Gastroenterol Hepatol. 2018 Aug 29;2018:8543763. doi: 10.1155/2018/8543763. eCollection 2018. Can J Gastroenterol Hepatol. 2018. PMID: 30228976 Free PMC article. Review.
Cited by
- A Gedunin-Type Limonoid, 7-Deacetoxy-7-Oxogedunin, from Andiroba (Carapa guianensis Aublet) Reduced Intracellular Triglyceride Content and Enhanced Autophagy in HepG2 Cells.
Nagatomo A, Ninomiya K, Marumoto S, Sakai C, Watanabe S, Ishikawa W, Manse Y, Kikuchi T, Yamada T, Tanaka R, Muraoka O, Morikawa T. Nagatomo A, et al. Int J Mol Sci. 2022 Oct 28;23(21):13141. doi: 10.3390/ijms232113141. Int J Mol Sci. 2022. PMID: 36361930 Free PMC article. - Salvianolic acid B from Salvia miltiorrhiza bunge: A potential antitumor agent.
Guo SS, Wang ZG. Guo SS, et al. Front Pharmacol. 2022 Oct 25;13:1042745. doi: 10.3389/fphar.2022.1042745. eCollection 2022. Front Pharmacol. 2022. PMID: 36386172 Free PMC article. Review. - tsRNA, cell death and disease: Connecting the dots.
Ma X, Xu J, Li G. Ma X, et al. Noncoding RNA Res. 2025 Apr 29;13:109-120. doi: 10.1016/j.ncrna.2025.04.006. eCollection 2025 Aug. Noncoding RNA Res. 2025. PMID: 40487298 Free PMC article. Review. - Autophagy and Senescence: The Molecular Mechanisms and Implications in Liver Diseases.
Li Q, Lin Y, Liang G, Xiao N, Zhang H, Yang X, Yang J, Liu A. Li Q, et al. Int J Mol Sci. 2023 Nov 28;24(23):16880. doi: 10.3390/ijms242316880. Int J Mol Sci. 2023. PMID: 38069199 Free PMC article. Review. - Epidemiologic, Genetic, Pathogenic, Metabolic, Epigenetic Aspects Involved in NASH-HCC: Current Therapeutic Strategies.
Gutiérrez-Cuevas J, Lucano-Landeros S, López-Cifuentes D, Santos A, Armendariz-Borunda J. Gutiérrez-Cuevas J, et al. Cancers (Basel). 2022 Dec 20;15(1):23. doi: 10.3390/cancers15010023. Cancers (Basel). 2022. PMID: 36612019 Free PMC article. Review.
References
- Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. - DOI - PubMed
- Kleiner D.E., Brunt E.M., Wilson L.A., Behling C., Guy C., Contos M., Cummings O., Yeh M., Gill R., Chalasani N., et al. Association of Histologic Disease Activity with Progression of Nonalcoholic Fatty Liver Disease. JAMA Netw. Open. 2019;2:e1912565. doi: 10.1001/jamanetworkopen.2019.12565. - DOI - PMC - PubMed
- Ludwig J., Viggiano T.R., McGill D.B., Oh B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980;55:434–438. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical