Role of HMGB1 in Cutaneous Melanoma: State of the Art - PubMed (original) (raw)
Review
Role of HMGB1 in Cutaneous Melanoma: State of the Art
Federica Li Pomi et al. Int J Mol Sci. 2022.
Abstract
High-mobility Group Box 1 (HMGB1) is a nuclear protein that plays a key role in acute and chronic inflammation. It has already been studied in several diseases, among them melanoma. Indeed, HMGB1 is closely associated with cell survival and proliferation and may be directly involved in tumor cell metastasis development thanks to its ability to promote cell migration. This research aims to assess the role of this molecule in the pathogenesis of human melanoma and its potential therapeutic role. The research has been conducted on the PubMed database, and the resulting articles are sorted by year of publication, showing an increasing interest in the last five years. The results showed that HMGB1 plays a crucial role in the pathogenesis of skin cancer, prognosis, and therapeutical response to therapy. Traditional therapies target this molecule indirectly, but future perspectives could include the development of new target therapy against HMGB1, thus adding a new approach to the therapy, which has often shown primary and secondary resistance. This could add a new therapy arm which has to be prolonged and specific for each patient.
Keywords: DAMPs; HMGB1; RAGE; alarmins; biomarkers; cancer; immunogenic cell death; melanoma; metastasis; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
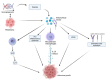
Figure 1
Tumor microenvironment and hypoxia, caused by cancer cells’ metabolic and oxygen demands, enhance HMGB1 release. HMGB1 binds to RAGE and actives several pathways: γδ-T cells, pro-inflammatory cytokines release, M2-macrophages, VEGF activation, and endothelial cell activation. All these lead to melanoma growth through the maintenance of an inflammatory microenvironment. Created with BioRender.com.
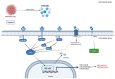
Figure 2
HMGB1 is released into the extracellular environment through a passive mechanism by melanoma cells. HMGB1 binds to RAGE, TLR2, and TLR4 and transduces cellular signals through a common pathway that induces the NF-κB pathway. The activated NF-κB translocates to the nucleus. HMGB1 also interacts with CXCR4 to activate the NF-κB pathway and induce chemotaxis and recruitment of inflammatory cells. The interaction of HMGB1 and TIM-3 induces the secretion of VEGF to promote tumor angiogenesis. All these pathways promote cell survival, cell proliferation and, finally, melanoma progression. Created with BioRender.com.
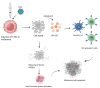
Figure 3
T-VEC is subcutaneously injected directly into the site of cancer. T-VEc attacks the melanoma cells locally, but also triggers the release of GM-CSF which recruits and actives dendritic cells. Dendritic cells activate CD8+ and CD4+ cells which, in turn, attack melanoma causing cell apoptosis and tumor reduction. Created with BioRender.com.
Similar articles
- Clinical Implications of High-mobility Group Box-1 (HMGB1) and the Receptor for Advanced Glycation End-products (RAGE) in Cutaneous Malignancy: A Systematic Review.
Nguyen AH, Detty SQ, Agrawal DK. Nguyen AH, et al. Anticancer Res. 2017 Jan;37(1):1-7. doi: 10.21873/anticanres.11282. Anticancer Res. 2017. PMID: 28011467 Review. - Targeting Inflammation Driven by HMGB1.
Yang H, Wang H, Andersson U. Yang H, et al. Front Immunol. 2020 Mar 20;11:484. doi: 10.3389/fimmu.2020.00484. eCollection 2020. Front Immunol. 2020. PMID: 32265930 Free PMC article. Review. - Ultraviolet light exposure stimulates HMGB1 release by keratinocytes.
Johnson KE, Wulff BC, Oberyszyn TM, Wilgus TA. Johnson KE, et al. Arch Dermatol Res. 2013 Nov;305(9):805-15. doi: 10.1007/s00403-013-1401-2. Epub 2013 Aug 13. Arch Dermatol Res. 2013. PMID: 23942756 Free PMC article. - High Mobility Group Box-1 (HMGb1): Current Wisdom and Advancement as a Potential Drug Target.
VanPatten S, Al-Abed Y. VanPatten S, et al. J Med Chem. 2018 Jun 28;61(12):5093-5107. doi: 10.1021/acs.jmedchem.7b01136. Epub 2018 Jan 5. J Med Chem. 2018. PMID: 29268019 Review. - The role of HMGB1 in inflammatory skin diseases.
Satoh TK. Satoh TK. J Dermatol Sci. 2022 Aug;107(2):58-64. doi: 10.1016/j.jdermsci.2022.07.005. Epub 2022 Jul 13. J Dermatol Sci. 2022. PMID: 35907655 Review.
Cited by
- Immunosenescence and Skin: A State of Art of Its Etiopathogenetic Role and Crucial Watershed for Systemic Implications.
Papa V, Li Pomi F, Borgia F, Vaccaro M, Pioggia G, Gangemi S. Papa V, et al. Int J Mol Sci. 2023 Apr 27;24(9):7956. doi: 10.3390/ijms24097956. Int J Mol Sci. 2023. PMID: 37175661 Free PMC article. Review. - Alarmins and MicroRNAs, a New Axis in the Genesis of Respiratory Diseases: Possible Therapeutic Implications.
Allegra A, Murdaca G, Gammeri L, Ettari R, Gangemi S. Allegra A, et al. Int J Mol Sci. 2023 Jan 16;24(2):1783. doi: 10.3390/ijms24021783. Int J Mol Sci. 2023. PMID: 36675299 Free PMC article. Review. - Ferroptosis in acute kidney injury following crush syndrome: A novel target for treatment.
Qiao O, Wang X, Wang Y, Li N, Gong Y. Qiao O, et al. J Adv Res. 2023 Dec;54:211-222. doi: 10.1016/j.jare.2023.01.016. Epub 2023 Jan 24. J Adv Res. 2023. PMID: 36702249 Free PMC article. Review. - Rosmarinus officinalis and Skin: Antioxidant Activity and Possible Therapeutical Role in Cutaneous Diseases.
Li Pomi F, Papa V, Borgia F, Vaccaro M, Allegra A, Cicero N, Gangemi S. Li Pomi F, et al. Antioxidants (Basel). 2023 Mar 9;12(3):680. doi: 10.3390/antiox12030680. Antioxidants (Basel). 2023. PMID: 36978928 Free PMC article. Review. - NETosis as an oncologic therapeutic target: a mini review.
Jaboury S, Wang K, O'Sullivan KM, Ooi JD, Ho GY. Jaboury S, et al. Front Immunol. 2023 Apr 18;14:1170603. doi: 10.3389/fimmu.2023.1170603. eCollection 2023. Front Immunol. 2023. PMID: 37143649 Free PMC article. Review.
References
- Garbe C., Amaral T., Peris K., Hauschild A., Arenberger P., Basset-Seguin N., Bastholt L., Bataille V., del Marmol V., Dréno B., et al. European Consensus-Based Interdisciplinary Guideline for Melanoma. Part 1: Diagnostics: Update 2022. Eur. J. Cancer. 2022;170:236–255. doi: 10.1016/j.ejca.2022.03.008. - DOI - PubMed
- Incorvaia L., Badalamenti G., Rinaldi G., Iovanna J.L., Olive D., Swayden M., Terruso L., Vincenzi B., Fulfaro F., Bazan V., et al. Can the Plasma PD-1 Levels Predict the Presence and Efficiency of Tumor-Infiltrating Lymphocytes in Patients with Metastatic Melanoma? Ther. Adv. Med. Oncol. 2019;11:1–15. doi: 10.1177/1758835919848872. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This research received no external funding.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical