Combined Risk Assessment of Food-derived Coumarin with in Silico Approaches - PubMed (original) (raw)
Combined Risk Assessment of Food-derived Coumarin with in Silico Approaches
Takashi Yamada et al. Food Saf (Tokyo). 2022.
Abstract
Hepatotoxicity associated with food-derived coumarin occurs occasionally in humans. We have, herein, assessed the data of existing clinical and nonclinical studies as well as those of in silico models for humans in order to shed more light on this association. The average intakes of food-derived coumarin are estimated to be 1-3 mg/day, while a ten-times higher level is expected in the worst-case scenarios. These levels are close to or above the tolerable daily intake suggested by a chronic study in dogs. The human internal exposure levels were estimated by a physiologically-based pharmacokinetic model with the use of virtual doses of coumarin in the amounts expected to derive from foods. Our results suggest that: (i) coumarin can be cleared rapidly via 7-hydroxylation in humans, and (ii) the plasma levels of coumarin and of its metabolite, _o_-hydroxyphenylacetic acid associated with hepatotoxicity, are considerably lower than those yielding hepatotoxicity in rats. Pharmacokinetic data suggest a low or negligible concern regarding a coumarin-induced hepatotoxicity in humans exposed to an average intake from foods. Detoxification of coumarin through the 7-hydroxylation, however, might vary among individuals due to genetic polymorphisms in CYP2A6 enzyme. In addition, the CYP1A2- and CYP2E1-mediated activation of coumarin can fluctuate as a result of induction caused by environmental factors. Furthermore, the daily consumption of food-contained coumarin was implicated in the potential risk of hepatotoxicity by the drug-induced liver injury score model developed by the US Food and Drug Administration. These results support the idea of the existence of human subpopulations that are highly sensitive to coumarin; therefore, a more precise risk assessment is needed. The present study also highlights the usefulness of in silico approaches of pharmacokinetics with the liver injury score model as battery components of a risk assessment.
Keywords: coumarin; drug-induced liver injury score model; hepatotoxicity; individual susceptibilities; physiologically based pharmacokinetics.
©2022 Food Safety Commission, Cabinet Office, Government of Japan.
Conflict of interest statement
Conflict of Interest: The authors have no conflict of interest regarding this publication.
Figures
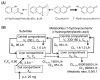
Fig. 1.
Major metabolic pathways of coumarin (A) and coumarin PBPK model (B). Input parameters for human coumarin PBPK model with two metabolites (7-hydroxycoumarin and_o_-HPA) were calculated previously). Values for fraction absorbed × intestinal availability (_F_a_F_g), absorption rate constant (_k_a), volume of the systemic circulation (_V_1), hepatic intrinsic clearance (_CL_h,int), hepatic clearance (_CL_h), and renal clearance (_CL_r) values for human PBPK models are shown._X_g represents the amount of compound in the gut compartment, _V_h represents liver volume,_C_h represents hepatic substrate concentration, and_C_b represents blood substrate concentration.
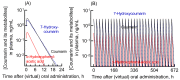
Fig. 2.
Plasma levels of coumarin and its metabolites in humans estimated using the established human PBPK model. The plasma concentrations of coumarin (black solid line), _o_-HPA (red solid line), and 7-hydroxycoumarin (blue dashed line) after virtual administration of 25 mg of coumarin via the oral route as a single dose (A) or daily doses for 28 days (B) are shown.
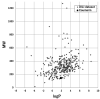
Fig. 3.
Application of coumarin to the FDA DILI score model. MW plotted against logP is presented for coumarin (black circle) and the set molecules for constructing the model, with a total of 354 substances (gray diamond).
Similar articles
- Metabolic profiles of coumarin in human plasma extrapolated from a rat data set with a simplified physiologically based pharmacokinetic model.
Miura T, Kamiya Y, Hina S, Kobayashi Y, Murayama N, Shimizu M, Yamazaki H. Miura T, et al. J Toxicol Sci. 2020;45(11):695-700. doi: 10.2131/jts.45.695. J Toxicol Sci. 2020. PMID: 33132243 - Dietary glycation compounds - implications for human health.
Hellwig M, Diel P, Eisenbrand G, Grune T, Guth S, Henle T, Humpf HU, Joost HG, Marko D, Raupbach J, Roth A, Vieths S, Mally A. Hellwig M, et al. Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724 - Metabolic activation and deactivation of dietary-derived coumarin mediated by cytochrome P450 enzymes in rat and human liver preparations.
Murayama N, Yamazaki H. Murayama N, et al. J Toxicol Sci. 2021;46(8):371-378. doi: 10.2131/jts.46.371. J Toxicol Sci. 2021. PMID: 34334558 - Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.
EFSA GMO Panel Working Group on Animal Feeding Trials. EFSA GMO Panel Working Group on Animal Feeding Trials. Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review. - CYP2A6 polymorphisms: is there a role for pharmacogenomics in preventing coumarin-induced hepatotoxicity in lymphedema patients?
Farinola N, Piller NB. Farinola N, et al. Pharmacogenomics. 2007 Feb;8(2):151-8. doi: 10.2217/14622416.8.2.151. Pharmacogenomics. 2007. PMID: 17286538 Review.
Cited by
- Application of CYP1A2-Template System to Understand Metabolic Processes in the Safety Assessment.
Murayama N, Yamada T, Yamazoe Y. Murayama N, et al. Food Saf (Tokyo). 2022 Dec 23;10(4):129-139. doi: 10.14252/foodsafetyfscj.D-22-00008. eCollection 2022 Dec. Food Saf (Tokyo). 2022. PMID: 36619007 Free PMC article. - Application of fused-grid-based CYP-Template systems for genotoxic substances to understand the metabolisms.
Yamazoe Y, Murayama N, Kawamura T, Yamada T. Yamazoe Y, et al. Genes Environ. 2023 Aug 7;45(1):22. doi: 10.1186/s41021-023-00275-4. Genes Environ. 2023. PMID: 37544994 Free PMC article.
References
- European Food Safety Authority. Coumarin in flavourings and other food ingredients with flavouring properties - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC). EFSA J. 2008; 6: 793. Available at https://www.efsa.europa.eu/en/efsajournal/pub/793. - PMC - PubMed
- Andréjak M,Gersberg M,Sgro C,et al. . French pharmacovigilance survey evaluating the hepatic toxicity of coumarin. Pharmacoepidemiol Drug Saf. 1998; 7(S1, Suppl 1): S45–S50. , 10.1002/(SICI)1099-1557(199808)7:1+S45::AID-PDS3533.0.CO;2-1 - DOI - PubMed