Systemic corticosteroids for radicular and non-radicular low back pain - PubMed (original) (raw)
Review
Systemic corticosteroids for radicular and non-radicular low back pain
Roger Chou et al. Cochrane Database Syst Rev. 2022.
Abstract
Background: Corticosteroids are medications with anti-inflammatory and immunosuppressant properties. Systemic corticosteroids administered through the oral, intravenous, or intramuscular routes have been used to treat various types of low back pain, including radicular back pain (not due to spinal stenosis), non-radicular back pain, and spinal stenosis. However, there is uncertainty about the benefits and harms of systemic corticosteroids for low back pain.
Objectives: To evaluate the benefits and harms of systemic corticosteroids versus placebo or no corticosteroid for radicular low back pain, non-radicular low back pain, and symptomatic spinal stenosis in adults.
Search methods: We used standard, extensive Cochrane search methods. The latest search date was September 2021.
Selection criteria: We included randomized and quasi-randomized trials in adults of systematic corticosteroids versus placebo or no corticosteroid.
Data collection and analysis: We used standard Cochrane methods. The major outcomes were pain, function, need for surgery, serious adverse effect, and presence of hyperglycemia. The minor outcomes were quality of life, successful outcomes, non-serious adverse events, and withdrawal due to adverse events. We used GRADE to assess the certainty of evidence for each outcome.
Main results: Thirteen trials (1047 participants) met the inclusion criteria. Nine trials included participants with radicular low back pain, two trial with low back pain, and two trials with spinal stenosis. All trials blinded participants to receipt of systemic corticosteroids. Seven trials were at low risk of bias, five at unclear risk, and one at high risk of selection bias. Two trials were at high risk of attrition bias. Doses and duration of systemic corticosteroid therapy varied. Radicular low back pain For radicular low back pain, moderate-certainty evidence indicated that systemic corticosteroids probably slightly decrease pain versus placebo at short-term follow-up (mean difference (MD) 0.56 points better, 95% confidence interval (CI) 1.08 to 0.04 on a 0 to 10 scale) and may slightly increase the likelihood of experiencing improvement in pain at short-term follow-up (risk ratio (RR) 1.21, 95% CI 0.88 to 1.66; absolute effect 5% better (95% CI 5% worse to 15% better). Systemic corticosteroids may not improve function at short-term follow-up (standardized mean difference (SMD) 0.14 better; range 0.49 better to 0.21 worse) and probably increase the likelihood of improvement in function at short-term follow-up (RR 1.52, 95% CI 1.22 to 1.91; absolute effect 19% better, 95% CI 8% better to 30% better). Systemic corticosteroids were associated with greater improvement in function versus placebo at long-term follow-up (MD -7.40, 95% CI -12.55 to -2.25 on the 0 to 100 Oswestry Disability Index) and greater likelihood of functional improvement (RR 1.29, 95% CI 1.06 to 1.56), based on a single trial. There was no difference in likelihood of surgery (RR 1.00, 95% CI 0.68 to 1.47). Evidence indicated that systemic corticosteroids (administered as a single dose or as a short course of therapy) are not associated with increased risk of any adverse event, serious adverse events, withdrawal due to adverse events, or hyperglycemia, but estimates were imprecise as some trials did not report harms, and harms reporting was suboptimal in trials that did provide data. Limitations included variability across trials in interventions (e.g. corticosteroid used, dose and duration of treatment), clinical settings, and participants (e.g. duration of symptoms, methods for diagnosis); limited utility of subgroup analyses due to small numbers of trials; methodologic limitations or suboptimal reporting of methods by some trials; and too few trials to formally assess for publication bias using graphical or statistical tests for small sample effects. Non-radicular low back pain Evidence on systemic corticosteroids versus placebo for non-radicular pain was limited and suggested that systemic corticosteroids may be associated with slightly worse short-term pain but slightly better function. Spinal stenosis For spinal stenosis, limited evidence indicated that systemic corticosteroids are probably no more effective than placebo for short-term pain or function.
Authors' conclusions: Systemic corticosteroids appear to be slightly effective at improving short-term pain and function in people with radicular low back pain not due to spinal stenosis, and might slightly improve long-term function. The effects of systemic corticosteroids in people with non-radicular low back pain are unclear and systemic corticosteroids are probably ineffective for spinal stenosis. A single dose or short course of systemic corticosteroids for low back pain does not appear to cause serious harms, but evidence is limited.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
RC has conducted systematic reviews funded by the Agency for Healthcare Research and Quality and the American Pain Society that included systemic corticosteroids, and he led a guideline from the American College of Physicians and the American Pain Society that addressed systemic corticosteroids.
RZP: none.
RF: Oregon Health & Science University (employment).
RL owns stocks in companies that make systemic corticosteroids (Pfizer Inc, Amgen, Eli Lilly and Company, Merck, Johnson & Johnson Health Care Systems Inc, Gilead Sciences). His declaration was referred to the Cochrane Funding Arbiter, and it was determined that there was no breach of Cochrane policy.
NH: none.
JHM: none
TD: none.
Figures
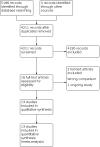
1
Study flow diagram.
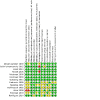
2
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
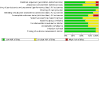
3
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
4
Corticosteroid versus placebo, immediate‐term (less than two weeks) pain (continuous).
5
Corticosteroid versus placebo, short‐term (two weeks to three months) pain (continuous).
6
Corticosteroid versus placebo, immediate‐term (less than two weeks) pain (dichotomous).
7
Corticosteroid versus placebo, short‐term (two weeks to three months) pain (dichotomous).
8
Corticosteroid versus placebo, intermediate‐term (greater than three to less than 12 months) pain (dichotomous).
9
Corticosteroid versus placebo, long‐term (12 months or greater) pain (dichotomous).
10
Corticosteroid versus placebo, immediate‐term (less than two weeks) function (continuous).
11
Corticosteroid versus placebo, short‐term (two weeks to three months) function (continuous).
12
Corticosteroid versus placebo, immediate‐term (less than two weeks) function (dichotomous).
13
Corticosteroid versus placebo, short‐term (two weeks to three months) function (dichotomous).
14
Corticosteroid versus placebo, long‐term (12 months or greater) function (dichotomous).
15
Corticosteroid versus placebo, subsequent surgery.
16
Corticosteroid versus placebo, hyperglycemia.
17
Corticosteroid versus placebo, immediate‐term (less than two weeks) global improvement.
18
Corticosteroid versus placebo, any adverse event.
19
Corticosteroid versus placebo, withdrawal due to adverse events.
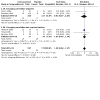
1.1. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 1: Pain (dichotomous), immediate (< 2 weeks) – by type of pain
1.2. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 2: Pain (dichotomous), short‐term (2 weeks to 3 months) – by type of pain
1.3. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 3: Pain (dichotomous), intermediate (> 3 to < 12 months)
1.4. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 4: Pain (dichotomous), long‐term (≥ 12 months)
1.5. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 5: Function (dichotomous), immediate (< 2 weeks) – by type of pain
1.6. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 6: Function (dichotomous), short‐term (2 weeks to 3 months) – by type of pain
1.7. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 7: Function (dichotomous), short‐term (radicular) – by corticosteroid dose
1.8. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 8: Function (dichotomous), short‐term (radicular) – by dosing regimen
1.9. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 9: Function (dichotomous), short‐term (radicular) – by route of administration
1.10. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 10: Function (dichotomous), short‐term (radicular) – by clinical setting
1.11. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 11: Function (dichotomous), short‐term (radicular) – by duration of symptoms
1.12. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 12: Function (dichotomous), short‐term (radicular) – by imaging correlation
1.13. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 13: Function (dichotomous), short‐term (radicular) – by risk of bias
1.14. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 14: Function (dichotomous), long‐term (≥12 months)
1.15. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 15: Surgery (radicular)
1.16. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 16: Surgery (radicular) – by risk of bias
1.17. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 17: Surgery (radicular) – by route of administration
1.18. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 18: Surgery (radicular) – by duration of symptoms
1.19. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 19: Surgery (radicular) – by imaging correlation
1.20. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 20: Serious adverse events
1.21. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 21: Hyperglycemia – by route of administration
1.22. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 22: Hyperglycemia – by type of pain
1.23. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 23: Hyperglycemia – by clinical setting
1.24. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 24: Hyperglycemia – by imaging correlation
1.25. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 25: Global improvement, immediate (< 2 weeks)
1.26. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 26: Global improvement, immediate (radicular) – by route of administration
1.27. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 27: Any adverse event – by duration of symptoms
1.28. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 28: Any adverse event – by type of pain
1.29. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 29: Any adverse event – by clinical setting
1.30. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 30: Any adverse event – by risk of bias
1.31. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 31: Any adverse event – by corticosteroid dose
1.32. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 32: Any adverse event – by imaging correlation
1.33. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 33: Any adverse event – by dosing regimen
1.34. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 34: Any adverse event – by route of administration
1.35. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 35: Gastrointestinal adverse events – by duration of symptoms
1.36. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 36: Gastrointestinal adverse events – by type of pain
1.37. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 37: Gastrointestinal adverse events – by clinical setting
1.38. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 38: Gastrointestinal adverse events – by corticosteroid dose
1.39. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 39: Gastrointestinal adverse events – by imaging correlation
1.40. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 40: Gastrointestinal adverse events – by dosing regimen
1.41. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 41: Gastrointestinal adverse events – by route of administration
1.42. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 42: Withdrawal due to adverse events
1.43. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 43: Withdrawal due to adverse events (radicular) – by duration of symptoms
1.44. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 44: Withdrawal due to adverse events (radicular) – by route of administration
1.45. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 45: Withdrawal due to adverse events (radicular) – by clinical setting
1.46. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 46: Withdrawal due to adverse events (radicular) – by dosing regimen
1.47. Analysis
Comparison 1: Corticosteroid versus placebo, Outcome 47: Withdrawal due to adverse events (radicular) – by imaging correlation
Comment in
- What Is the Role of Systemic Corticosteroids for Radicular and Nonradicular Low Back Pain?
Gottlieb M, Fagan MW, Polich ED. Gottlieb M, et al. Ann Emerg Med. 2023 Aug;82(2):164-166. doi: 10.1016/j.annemergmed.2023.01.007. Epub 2023 Feb 15. Ann Emerg Med. 2023. PMID: 36797131 No abstract available. - Benefits and Harms of Systemic Corticosteroids for Radicular and Nonradicular Low Back Pain.
Leggit JC. Leggit JC. Am Fam Physician. 2023 Jun;107(6):583-584. Am Fam Physician. 2023. PMID: 37327157 No abstract available.
Similar articles
- Epidural corticosteroid injections for lumbosacral radicular pain.
Oliveira CB, Maher CG, Ferreira ML, Hancock MJ, Oliveira VC, McLachlan AJ, Koes BW, Ferreira PH, Cohen SP, Pinto RZ. Oliveira CB, et al. Cochrane Database Syst Rev. 2020 Apr 9;4(4):CD013577. doi: 10.1002/14651858.CD013577. Cochrane Database Syst Rev. 2020. PMID: 32271952 Free PMC article. - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - Non-steroidal anti-inflammatory drugs for acute low back pain.
van der Gaag WH, Roelofs PD, Enthoven WT, van Tulder MW, Koes BW. van der Gaag WH, et al. Cochrane Database Syst Rev. 2020 Apr 16;4(4):CD013581. doi: 10.1002/14651858.CD013581. Cochrane Database Syst Rev. 2020. PMID: 32297973 Free PMC article. - Yoga treatment for chronic non-specific low back pain.
Wieland LS, Skoetz N, Pilkington K, Vempati R, D'Adamo CR, Berman BM. Wieland LS, et al. Cochrane Database Syst Rev. 2017 Jan 12;1(1):CD010671. doi: 10.1002/14651858.CD010671.pub2. Cochrane Database Syst Rev. 2017. PMID: 28076926 Free PMC article. Updated. Review. - Arthroscopic surgery for degenerative knee disease (osteoarthritis including degenerative meniscal tears).
O'Connor D, Johnston RV, Brignardello-Petersen R, Poolman RW, Cyril S, Vandvik PO, Buchbinder R. O'Connor D, et al. Cochrane Database Syst Rev. 2022 Mar 3;3(3):CD014328. doi: 10.1002/14651858.CD014328. Cochrane Database Syst Rev. 2022. PMID: 35238404 Free PMC article. Review.
Cited by
- Pharmacological treatments for low back pain in adults: an overview of Cochrane Reviews.
Cashin AG, Wand BM, O'Connell NE, Lee H, Rizzo RR, Bagg MK, O'Hagan E, Maher CG, Furlan AD, van Tulder MW, McAuley JH. Cashin AG, et al. Cochrane Database Syst Rev. 2023 Apr 4;4(4):CD013815. doi: 10.1002/14651858.CD013815.pub2. Cochrane Database Syst Rev. 2023. PMID: 37014979 Free PMC article. Review. - The challenge of pharmacotherapy for musculoskeletal pain: an overview of unmet needs.
Moretti A, Snichelotto F, Liguori S, Paoletta M, Toro G, Gimigliano F, Iolascon G. Moretti A, et al. Ther Adv Musculoskelet Dis. 2024 May 23;16:1759720X241253656. doi: 10.1177/1759720X241253656. eCollection 2024. Ther Adv Musculoskelet Dis. 2024. PMID: 38799611 Free PMC article. Review. - Epidural Injection of Harpagoside for the Recovery of Rats with Lumbar Spinal Stenosis.
Hong JY, Kim H, Yeo C, Lee J, Jeon WJ, Lee YJ, Ha IH. Hong JY, et al. Cells. 2023 Sep 15;12(18):2281. doi: 10.3390/cells12182281. Cells. 2023. PMID: 37759506 Free PMC article. - Analgesia for non-specific low back pain.
Jones CMP, Underwood M, Chou R, Schoene M, Sabzwari S, Cavanagh J, Lin CC. Jones CMP, et al. BMJ. 2024 Jun 27;385:e080064. doi: 10.1136/bmj-2024-080064. BMJ. 2024. PMID: 38936847 Free PMC article. No abstract available. - Can resistance exercise prevent breast cancer-related lymphoedema? A systematic review and metanalysis protocol.
Aguilera-Eguía RA, Seron P, Gutiérrez-Arias R, Zaror C. Aguilera-Eguía RA, et al. BMJ Open. 2024 Nov 19;14(11):e080935. doi: 10.1136/bmjopen-2023-080935. BMJ Open. 2024. PMID: 39566933 Free PMC article.
References
References to studies included in this review
Akbari Aghdam 2020 {published data only}
- Akbari Aghdam H, Andalib A, Adadiyan Ardakani H, Telloo M, Sheikhbahaei E. A short-term oral corticosteroid for refractory lumbar spinal stenosis: a double-blinded randomized placebo-controlled trials. International Journal of Rehabilitation Research 2020;43:342-6. - PubMed
Balakrishnamoorthy 2015 {published data only}
- Balakrishnamoorthy R, Horgan I, Perez S, Keijzers G, Steele M. Steroids for emergency patients with low back pain and radiculopathy (SEBRA): the SEBRA trial. EMA – Emergency Medicine Australasia 2013;25(S1):17.
- Balakrishnamoorthy R, Horgan I, Perez S, Steele MC, Keijzers GB. Does a single dose of intravenous dexamethasone reduce Symptoms in Emergency department patients with low Back pain and RAdiculopathy (SEBRA)? A double-blind randomised controlled trial. Emergency Medicine Journal 2015;32:525-30. - PubMed
Eskin 2014 {published data only}
- Eskin B, Shih RD, Allegra JR, Fiesseler FW, Silverman M. Steroids for back pain: a randomized double-blind placebo-controlled trial. Academic Emergency Medicine 2010;17:s154.
- Eskin B, Shih RD, Fiesseler FW, Walsh BW, Allegra JR, Silverman ME, et al. Prednisone for emergency department low back pain: a randomized controlled trial. Journal of Emergency Medicine 2014;47(1):65-70. - PubMed
Finckh 2006 {published and unpublished data}
- Finckh A, Zufferey P, Schurch MA, Balague F, Waldburger M, So AK. Short-term efficacy of intravenous pulse glucocorticoids in acute discogenic sciatica. Spine 2006;31:377-81. - PubMed
Friedman 2006 {published data only}
- Friedman BW, Holden L, Esses D, Bijur PE, Choi HK, Solorzano C, et al. Parenteral corticosteroids for emergency department patients with non-radicular low back pain. Journal of Emergency Medicine 2006;31:365-70. - PubMed
Friedman 2008 {published data only}
Goldberg 2015 {published data only}
Haimovic 1986 {published data only}
- Haimovic IC, Beresford HR. Dexamethasone is not superior to placebo for treating lumbosacral radicular pain. Neurology 1986;36:1593-4. - PubMed
- Haimovic IC, Beresford HR. Treatment of lumbosacral radicular pain with dexamethasone: a controlled study. Neurology 1985;35(Suppl 1):252.
Hedeboe 1982 {published data only}
- Hedeboe J, Buhl M, Ramsing P. Effects of using dexamethasone and placebo in the treatment of prolapsed lumbar disc. Acta Neurologica Scandinavica 1982;65:6-10. - PubMed
Hofferberth 1982 {published data only}
- Hofferberth B, Gottschaldt M, Grass H, Bűttner K. The usefulness of dexamethasonephosphate in the conservative treatment of lumbar pain – a double-blind study [Űber den wert der anwendung von dexamethasonphosphat in der konservativen therapie der lumboischialgie]. Archiv fűr Psychiatrie und Nervenkrankheiten 1982;231:359-67. - PubMed
Holve 2008 {published data only}
- Holve RL, Barkan H. Oral steroids in initial treatment of acute sciatica. Journal of the American Board of Family Medicine 2008;21:469-74. - PubMed
Porsman 1979 {published data only}
- Porsman O, Friis H. Prolapsed lumbar disc treated with intramuscularly administered dexamethasone phosphate. Scandinavian Journal of Rheumatology 1979;8:142-4. - PubMed
Rodrigues 2014 {published data only}
References to studies excluded from this review
Ko 2016 {published data only}
Oros 2019 {published data only}
References to ongoing studies
Liu 2020 {published data only}
Additional references
Abdel Shaheed 2019
- Abdel Shaheed C, Maher CG, Buchbinder R, Ng B, Enke O, Guzowski R, et al. Efficacy and harms of orally, intramuscularly, or intravenously administered glucocorticoids for sciatica: a systematic review and meta-analysis. European Journal of Pain 2019;24:518-35. - PubMed
Carey 2009
Chiarotto 2016
- Chiarotto A, Maxwell LJ, Terwwee CB, Wells GA, Tugwell P, Ostelo RW. Roland-Morris Disability Questionnaire and Oswestry Disability Index: which has better measurement properties for measuring physical functioning in nonspecific low back pain? Systematic review and meta-analysis. Physical Therapy 2016;96(10):1620-37. [PMID: ] - PubMed
Chou 2007
- Chou R, Qaseem A, Snow V, Casey D, Cross JT Jr, Shekelle P, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Annals of Internal Medicine 2007;147(7):478-91. - PubMed
Chou 2011
- Chou R, Qaseem A, Owens DK, Shekelle P, Clinical Guidelines Committee of the American College of Physicians. Diagnostic imaging for low back pain: advice for high-value health care from the American College of Physicians. Annals of Internal Medicine 2011;154(3):181-9. - PubMed
Chou 2015
- Chou R, Hashimoto R, Friedly J, Fu R, Bougatsos C, Dana T, et al. Epidural corticosteroid injections for radiculopathy and spinal stenosis: a systematic review and meta-analysis. Annals of Internal Medicine 2015;163(5):373-81. - PubMed
Chrousos 2014
- Chrousos GP. Chapter 39. Adrenal corticosteroids & adrenocortical antagonists. In: Katzung BG, Masters SB, Trevor AJ, editors(s). Basic & Clinical Pharmacology. 12th edition. New York (NY): McGraw-Hill Companies, Inc, 2012:697-714.
da C Menezes Costa 2012
Dekker 2019
- Dekker J. The minimal clinically important difference re-considered. Osteoarthritis and Cartilage 2019;27(10):1403-4. - PubMed
Dexter 1995
- Dexter F, Chestnut DH. Analysis of statistical tests to compare visual analog scale measurements among groups. Anesthesiology 1995;82:896-902. [PMID: ] - PubMed
Deyo 2006
- Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine 2006;31(23):2724-7. - PubMed
Deyo 2014
Ferreira 2021
Friedman 2010
Fu 2016
- Fu R, Holmer H. Change score or followup score? Choice of mean difference estimates could impact meta-analysis conclusions. Journal of Clinical Epidemiology 2016;76:108-17. - PubMed
Furlan 2015
- Furlan AD, Pennick V, Bombardier C, Tulder M. 2015 updated method guidelines for systematic reviews in the Cochrane Back and Neck Group. Spine 2015;40(21):1660-73. - PubMed
Green 1975
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.traintraining.cochrane.org/handbook/archive/v6.2. - PubMed
Hoy 2012
- Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, et al. A systematic review of the global prevalence of low back pain. Arthritis & Rheumatism 2012;64(6):2028-37. - PubMed
Jarvik 2002
- Jarvik JG, Deyo RA. Diagnostic evaluation of low back pain with emphasis on imaging. Annals of Internal Medicine 2002;137(7):586-97. - PubMed
Kopec 2000
- Kopec JA. Measuring functional outcomes in persons with back pain: a review of back-specific questionnaires. Spine 2000;25(24):3110-4. [PMID: ] - PubMed
Luo 2004
- Luo X, Pietroban R, Sun SX, Liu GG, Hey L. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine 2004;29(1):79-86. - PubMed
Machado 2017
- Machado GC, Maher CG, Ferreira PH, O Day R, Pinheiro MB, Ferreira ML. Non-steroidal anti-inflammatory drugs for spinal pain: a systematic review and meta-analysis. Annals of the Rheumatic Diseases 2017;76:1269-78. - PubMed
Martin 2008
- Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, et al. Expenditures and health status among adults with back and neck problems. JAMA 2008;299(6):656-64. - PubMed
McDonough 2008
- McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Current Opinion in Rheumatology 2008;20(2):131-7. - PubMed
Oliveira 2020
- Oliveira CB, Maher CG, Ferreira ML, Hancock MJ, Oliveira VC, McLachlan AJ, et al. Epidural corticosteroid injections for sciatica: an abridged Cochrane systematic review and meta-analysis. Spine 2020;45(21):E1405-15. - PubMed
Ostelo 2008
- Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Korff M, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine 2008;33(1):90-4. - PubMed
Qaseem 2017
- Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Annals of Internal Medicine 2017;166:514-30. - PubMed
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Roncoroni 2011
- Roncoroni C, Baillet A, Durand M, Gaudin P, Juvin R. Efficacy and tolerance of systemic steroids in sciatica: a systematic review and meta-analysis. Rheumatology 2011;50(9):1603-11. - PubMed
Ropper 2015
- Ropper AH, Zafonte RD. Sciatica. New England Journal of Medicine 2015;372(13):1240-8. - PubMed
Rubinstein 2011
Salas Apaza 2021
- Salas Apaza JA, Franco JV, Meza N, Madrid E, Loézar C, Garegnani L. Minimal clinically important difference: the basics. Medwave 2021;21(3):e8149. - PubMed
Schimmer 2011
- Schimmer BP, Funder JW. Chapter 42. ACTH, adrenal steroids, and pharmacology of the adrenal cortex. In: Brunton LL, Chabner BA, Knollmann BC, editors(s). Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12th edition. New York (NY): McGraw-Hill Companies, Inc, 2011:1209-36.
Sedaghat 2019
- Sedaghat AR. Understanding the minimal clinically important difference (MCID) of patient-reported outcome measures. Otolaryngology – Head and Neck Surgery 2019;161(4):551-60. - PubMed
Shafshak 2021
- Shafshak TS, Elnemr R. The visual analogue scale versus numerical rating scale in measuring pain severity and predicting disability in low back pain. Journal of Clinical Rheumatology 2021;27(7):282-5. [PMID: ] - PubMed
Stata [Computer program]
- Stata. Version 14. College Station, TX, USA: StataCorp, 2015. Available at www.stata.com.
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. - PubMed
van der Laan 2008
- Laan S, Meijer OC. Pharmacology of glucocorticoids: beyond receptors. European Journal of Pharmacology 2008;585(2-3):483-91. - PubMed
Vickers 2005
References to other published versions of this review
Chou 2016
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous