Opportunities and short-comings of the axolotl salamander heart as a model system of human single ventricle and excessive trabeculation - PubMed (original) (raw)
Opportunities and short-comings of the axolotl salamander heart as a model system of human single ventricle and excessive trabeculation
Sophie Meyer et al. Sci Rep. 2022.
Abstract
Few experimental model systems are available for the rare congenital heart diseases of double inlet left ventricle (DILV), a subgroup of univentricular hearts, and excessive trabeculation (ET), or noncompaction. Here, we explore the heart of the axolotl salamander (Ambystoma mexicanum, Shaw 1789) as model system of these diseases. Using micro-echocardiography, we assessed the form and function of the heart of the axolotl, an amphibian, and compared this to human DILV (n = 3). The main finding was that both in the axolotl and DILV, blood flows of disparate oxygen saturation can stay separated in a single ventricle. In the axolotl there is a solitary ventricular inlet and outlet, whereas in DILV there are two separate inlets and outlets. Axolotls had a lower resting heart rate compared to DILV (22 vs. 72 beats per minute), lower ejection fraction (47 vs. 58%), and their oxygen consumption at rest was higher than peak oxygen consumption in DILV (30 vs. 17 ml min-1 kg-1). Concerning the ventricular myocardial organization, histology showed trabeculations in ET (n = 5) are much closer to the normal human setting than to the axolotl setting. We conclude that the axolotl heart resembles some aspects of DILV and ET albeit substantial species differences exist.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Figure 1
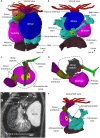
Anatomy of the axolotl heart. (A) Ventral view of the in situ axolotl heart showing the principle chambers, namely sinus venosus, atrium (internally divided by an almost complete atrial septum), ventricle and conus arteriosus. The ventricle is positioned on the right and the blunt apex is positioned caudally. The arterial outflow tract is a solitary trunk (truncus arteriosus) with left–right paired channels, giving rise to the third (3rd), fourth (4th), fifth (5th) and sixth (6th) gill arch arteries. There is no diaphragm between the heart and liver. (B) Dorsal view, showing the presence of a left sinus horn. Notice that a solitary pulmonary vein connects to the atrium in a position that equals the human embryonic position, i.e. immediately cranial to the sinus venosus and to the left of the sinuatrial junction. (C) View of the ventricular base and the atrial septum. The pulmonary vein connects to the left atrium in a primitive position, i.e. close to the ventricular base and next to the atrial septum. (D) Left-sided view of the atrial septum, showing a small gap between its leading edge and the atrioventricular canal, i.e. the primary foramen of the atrial septum. (E) Image from microCT showing the highly trabeculated state of the ventricle. Notice there is no ventricular septum, there is a solitary atrioventricular canal (white arrowheads) and a common orifice to the outflow tract (black arrowheads). (F) Ventral-left view of the heart without the atrium (its outline is indicated by the dashed line). Notice the central position of the solitary atrioventricular canal and the cranial-right position of the outflow tract (conus arteriosus). Such topology resembles a double-inlet left ventricle with double-outlet right ventricle. eso, esophagus.
Figure 2
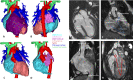
Anatomy of double inlet left ventricle patient 2. (A) Anterior view, complete. (B) Posterior view, complete. (C) Anterior view, right ventricle removed. (D) Both ventricles removed, appreciate anterior/posterior relationship of atria. (E) 4-chamber view. (F) Pathway of oxygen-poor blood stream. (G) Superior-inferior relationship of the atria. (H) Pathway of oxygen-rich blood stream. VSD, ventricular septal defect.
Figure 3
Anatomy and 4D streaming analysis of double inlet left ventricle patient 3. (A) 3-chamber view. (B) Sagittal view. (C) Coronal view. (D) Early ventricular filling phase depicting oxygen-poor (blue) blood and oxygen-rich (red) blood streams entering the left ventricle through the right and left atrioventricular valves respectively. (E) Late ventricular filling phase depicting oxygen-poor (blue) blood and oxygen-rich (red) blood streams entering the left ventricle through the right and left atrioventricular valves respectively. (F) Ventricular ejection phase depicting oxygen-poor (blue) blood and oxygen-rich (red) blood streams entering the pulmonary artery and aorta respectively. Oxygen-poor blood is not ejected in the aorta, blood ejected into the pulmonary artery seems partially oxygen-rich and oxygen-poor. (G) Ejection of blood in aorta (left) and pulmonary artery (right).
Figure 4
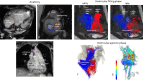
Cardiac flow separation in the axolotl demonstrated with ultrasonography. Top left: Anatomical model of the gill arches in the axolotl (3rd arch red, 4th arch purple, 5th arch green, 6th arch blue). Top right: Ultrasonographic transversal cross sections in the outflow tract at four time points in the injection series of negative contrast agent (amphibian Ringer’s solution). Notice the signal decrease (arrows) as diluted blood perfuse the three ventral gill arches after 30 and 3 cardiac cycles of contrast agent infusion at 0.5 and 5 ml min−1 respectively. Main part: Signal traces in right and left arches over time. Traces on the right are magnifications of the last 63 cardiac cycles with a high rate of infusion of contrast agent. Notice the final signal decrease in the 3rd, 4th, and 5th gill arches supplying the gills but not in the 6th gill arch supplying the lungs indicating a lack of complete mixing of blood in the heart as hypointense contrast agent is injected into the sinus venosus at a high flow rate (5ml min-1).
Figure 5
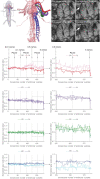
Ventricular wall composition in amphibians and human with normal and excessively trabeculated left ventricle. (A,B) Transverse section of the ventricular base of an axolotl (A) and a Xenopus frog (B), showing that the ventricle is highly trabecular and the compact wall is extremely thin. It can also be seen that the solitary atrioventricular canal (AVC) is left-sided and the solitary outflow tract (OFT) is right-sided. (C) Transverse section of the human ventricles at gestational week 20. This heart is not much bigger than the Xenopus heart (B), but the compact wall is much thicker and the trabeculations are much fewer and greater in size. (D) Transverse section of a case of fetal human excessive trabeculation at gestational week 20. Compared to the heart shown in (C), the compact wall is thinner and the trabeculations are more numerous, but this heart is still much different from the amphibian hearts (A–B). (E) Transmural histology of a normal left ventricle of an adult human, showing a thick compact wall and a few trabeculations. (F) Transmural histology of a case of left ventricular excessive trabeculation of an adult human. Compared to the heart shown in (E), the compact wall is thinner and the trabeculations are more numerous, but this wall composition is still much different from that of the amphibian hearts (A–B). (G) Measurements of the width of the compact wall (above the horizontal axis) and trabeculations (below the horizontal axis), shown on a logarithmic scale. Letters refer to the images (A–F) on which the measurements were done. (H) Counting of trabeculations per mm2 on images (A–F) showing that the amphibians have a 1–2 orders greater number of trabeculations per area. Staining is Masson’s trichrome in (A), picro-sirius red in (B, E and F), and hematoxylin–eosin in (C and D). The sections shown in (C,D) were part of the data published in.
Similar articles
- Banding differences between tiger salamander and axolotl chromosomes.
Cuny R, Malacinski GM. Cuny R, et al. Can J Genet Cytol. 1985 Oct;27(5):510-4. doi: 10.1139/g85-076. Can J Genet Cytol. 1985. PMID: 4063874 - 2D and 3D Echocardiography in the Axolotl (Ambystoma Mexicanum).
Dittrich A, Thygesen MM, Lauridsen H. Dittrich A, et al. J Vis Exp. 2018 Nov 29;(141). doi: 10.3791/57089. J Vis Exp. 2018. PMID: 30582577 - Myocardial cell relationships during morphogenesis in normal and cardiac lethal mutant axolotls, Ambystoma mexicanum.
Fransen ME, Lemanski LF. Fransen ME, et al. Am J Anat. 1988 Nov;183(3):245-57. doi: 10.1002/aja.1001830307. Am J Anat. 1988. PMID: 3213830 - Forever young: Endocrinology of paedomorphosis in the Mexican axolotl (Ambystoma mexicanum).
De Groef B, Grommen SVH, Darras VM. De Groef B, et al. Gen Comp Endocrinol. 2018 Sep 15;266:194-201. doi: 10.1016/j.ygcen.2018.05.016. Epub 2018 May 16. Gen Comp Endocrinol. 2018. PMID: 29777689 Review. - The new concept of univentricular heart.
Frescura C, Thiene G. Frescura C, et al. Front Pediatr. 2014 Jul 7;2:62. doi: 10.3389/fped.2014.00062. eCollection 2014. Front Pediatr. 2014. PMID: 25072035 Free PMC article. Review.
Cited by
- Ultrasound description of the coelomic cavity of the axolotl (Ambystoma mexicanum) in a clinically healthy population: a pilot study.
Vieu S, Le Poul N, Tur L, Aupée C, Kerbrat-Copy R, Bouhsina N, Cojean O, Fusellier M. Vieu S, et al. Sci Rep. 2024 May 23;14(1):11787. doi: 10.1038/s41598-024-62264-z. Sci Rep. 2024. PMID: 38782987 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous