Anticandidal Activity of Capsaicin and Its Effect on Ergosterol Biosynthesis and Membrane Integrity of Candida albicans - PubMed (original) (raw)
Anticandidal Activity of Capsaicin and Its Effect on Ergosterol Biosynthesis and Membrane Integrity of Candida albicans
Jawad M Behbehani et al. Int J Mol Sci. 2023.
Abstract
Oral candidiasis is an infection of the oral cavity commonly caused by Candida albicans. Endodontic treatment failure has also been found to be persistent from C. albicans in the root canal system. Despite the availability of antifungal drugs, the management of Candida oral infection is difficult as it exhibits resistance to a different class of antifungal drugs. Therefore, it is necessary to discover new antifungal compounds to cure fungal infections. This study aimed to examine the antifungal susceptibility of Capsaicin, an active compound of chili pepper. The susceptibility of Capsaicin and Fluconazole was tested against the Candida species by the CLSI (M27-A3) method. The effect of Capsaicin on the fungal cell wall was examined by the ergosterol inhibitory assay and observed by the scanning electron micrograph. The MIC range of Capsaicin against Candida isolates from oral (n = 30), endodontic (n = 8), and ATCC strains (n = 2) was 12.5−50 µg/mL. The MIC range of Fluconazole (128- 4 µg/mL) significantly decreased (2- to 4-fold) after the combination with Capsaicin (MIC/4) (p < 0.05). Capsaicin (at MIC) significantly reduced the mature biofilm of C. albicans by 70 to 89% (p < 0.01). The ergosterol content of the cell wall decreased significantly with the increase in the Capsaicin dose (p < 0.01). Capsaicin showed high sensitivity against the hyphae formation and demonstrated a more than 71% reduction in mature biofilm. A fluorescence microscopy revealed the membrane disruption of Capsaicin-treated C. albicans cells, whereas a micrograph of electron microscopy showed the distorted cells’ shape, ruptured cell walls, and shrinkage of cells after the release of intracellular content. The results conclude that Capsaicin had a potential antifungal activity that inhibits the ergosterol biosynthesis in the cell wall, and therefore, the cells’ structure and integrity were disrupted. More importantly, Capsaicin synergistically enhanced the Fluconazole antifungal activity, and the synergistic effect might be helpful in the prevention of Fluconazole resistance development and reduced drug-dosing.
Keywords: Capsaicin; anticandidal; chili pepper; ergosterol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
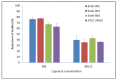
Figure 1
Effect of Capsaicin on the mature biofilm of C. albicans. The standard deviations (SD) of each sample are shown in the graph. The mean differences between the control and test molecules at the MIC level were statistically significant (_p_-level, 0.01). MIC/2—half of the MIC concentration; Endo—endodontic isolates.
Figure 2
Effect of Capsaicin (MIC) on the hyphae growth of C. albicans (Endo-902). The live cells photograph was recorded by the cell observer microscope (20×). The photograph of untreated cells (1a–1d) and treated cells (2a–2d) were recorded at three-hour time points, respectively.
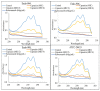
Figure 3
Spectrophotometric sterol profiles of C. albicans (Endo-904, Endo-906, and Endo-908). Sterols were extracted from the cells and spectral profiles between 230 and 300 nm were determined.
Figure 4
Confocal scanning laser microscopy (CSLM) images of endodontic C. albicans cells (Endo) treated with Capsaicin (MIC) (right panel) and untreated control cells (left panel). To observe membrane damage, cells were stained with propidium iodide (red signals).
Figure 5
Scanning electron micrograph of C. albicans cells with and without treatment with Capsaicin (MIC). Attachments of Candida cells (Endo-902 (A), Endo-903 (B), and Endo-904 (C)) with teeth pulp in without treatment condition are shown in left panel, whereas with treatment condition (Endo-902 (D), Endo-903 (E) and Endo-904 (F)) are shown in right panel.
Similar articles
- Synergistic effects of tea polyphenol epigallocatechin 3-O-gallate and azole drugs against oral Candida isolates.
Behbehani JM, Irshad M, Shreaz S, Karched M. Behbehani JM, et al. J Mycol Med. 2019 Jun;29(2):158-167. doi: 10.1016/j.mycmed.2019.01.011. Epub 2019 Feb 20. J Mycol Med. 2019. PMID: 30797684 - Antifungal Efficacy of Ultrashort β-Peptides against Candida Species: Mechanistic Understanding and Therapeutic Implications.
Kantroo HA, Mubarak MM, Chowdhary R, Rai R, Ahmad Z. Kantroo HA, et al. ACS Infect Dis. 2024 Nov 8;10(11):3736-3743. doi: 10.1021/acsinfecdis.4c00476. Epub 2024 Oct 11. ACS Infect Dis. 2024. PMID: 39392829 - Molecular epidemiology, antifungal susceptibility, and ERG11 gene mutation of Candida species isolated from vulvovaginal candidiasis: Comparison between recurrent and non-recurrent infections.
Esfahani A, Omran AN, Salehi Z, Shams-Ghahfarokhi M, Ghane M, Eybpoosh S, Razzaghi-Abyaneh M. Esfahani A, et al. Microb Pathog. 2022 Sep;170:105696. doi: 10.1016/j.micpath.2022.105696. Epub 2022 Jul 31. Microb Pathog. 2022. PMID: 35921954 Review. - Novel Preclinical Study of Galloylquinic Acid Compounds from Copaifera lucens with Potent Antifungal Activity against Vaginal Candidiasis Induced in a Murine Model via Multitarget Modes of Action.
Al-Madboly LA, Abd El-Salam MA, Bastos JK, El-Shorbagy SH, El-Morsi RM. Al-Madboly LA, et al. Microbiol Spectr. 2022 Oct 26;10(5):e0272421. doi: 10.1128/spectrum.02724-21. Epub 2022 Aug 16. Microbiol Spectr. 2022. PMID: 35972130 Free PMC article. - Candida and candidaemia. Susceptibility and epidemiology.
Arendrup MC. Arendrup MC. Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
Cited by
- Chemical composition and antifungal activity of Capsicum pepper aqueous extracts against plant pathogens and food spoilage fungi.
Sepúlveda M, Costa J, Cayún Y, Gallardo V, Barría E, Rigotto Caruso G, von Zeska Kress MR, Cornejo P, Santos C. Sepúlveda M, et al. Front Cell Infect Microbiol. 2024 Oct 3;14:1451287. doi: 10.3389/fcimb.2024.1451287. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39421640 Free PMC article. - Capsaicin: Emerging Pharmacological and Therapeutic Insights.
Petran EM, Periferakis A, Troumpata L, Periferakis AT, Scheau AE, Badarau IA, Periferakis K, Caruntu A, Savulescu-Fiedler I, Sima RM, Calina D, Constantin C, Neagu M, Caruntu C, Scheau C. Petran EM, et al. Curr Issues Mol Biol. 2024 Jul 24;46(8):7895-7943. doi: 10.3390/cimb46080468. Curr Issues Mol Biol. 2024. PMID: 39194685 Free PMC article. Review. - A comprehensive review of integrated management strategies for damping-off disease in chili.
Delai C, Muhae-Ud-Din G, Abid R, Tian T, Liu R, Xiong Y, Ma S, Ghorbani A. Delai C, et al. Front Microbiol. 2024 Oct 17;15:1479957. doi: 10.3389/fmicb.2024.1479957. eCollection 2024. Front Microbiol. 2024. PMID: 39483761 Free PMC article. Review. - Antimicrobial Properties of Capsaicin: Available Data and Future Research Perspectives.
Periferakis AT, Periferakis A, Periferakis K, Caruntu A, Badarau IA, Savulescu-Fiedler I, Scheau C, Caruntu C. Periferakis AT, et al. Nutrients. 2023 Sep 22;15(19):4097. doi: 10.3390/nu15194097. Nutrients. 2023. PMID: 37836381 Free PMC article. Review. - Bioturbation analysis of microbial communities and flavor metabolism in a high-yielding cellulase Bacillus subtilis biofortified Daqu.
Liu Y, Li H, Liu W, Ren K, Li X, Zhang Z, Huang R, Han S, Hou J, Pan C. Liu Y, et al. Food Chem X. 2024 Apr 16;22:101382. doi: 10.1016/j.fochx.2024.101382. eCollection 2024 Jun 30. Food Chem X. 2024. PMID: 38665634 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous