COF-based artificial probiotic for modulation of gut microbiota and immune microenvironment in inflammatory bowel disease - PubMed (original) (raw)
. 2022 Dec 27;14(6):1598-1605.
doi: 10.1039/d2sc04984h. eCollection 2023 Feb 8.
Affiliations
- PMID: 36794177
- PMCID: PMC9906670
- DOI: 10.1039/d2sc04984h
COF-based artificial probiotic for modulation of gut microbiota and immune microenvironment in inflammatory bowel disease
Qingqing Deng et al. Chem Sci. 2022.
Abstract
Conventional strategies for treating inflammatory bowel disease merely relieve inflammation and excessive immune response, but fail to solve the underlying causes of IBD, such as disrupted gut microbiota and intestinal barrier. Recently, natural probiotics have shown tremendous potential for the treatment of IBD. However, probiotics are not recommended for IBD patients, as they may cause bacteremia or sepsis. Herein, for the first time, we constructed artificial probiotics (Aprobiotics) based on artificial enzyme-dispersed covalent organic frameworks (COFs) as the "organelle" and a yeast shell as the membrane of the Aprobiotics to manage IBD. The COF-based artificial probiotics, with the function of natural probiotics, could markedly relieve IBD by modulating the gut microbiota, suppressing intestinal inflammation, protecting the intestinal epithelial cells, and regulating immunity. This nature-inspired approach may aid in the design of more artificial systems for the treatment of various incurable diseases, such as multidrug-resistant bacterial infection, cancer, and others.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
All authors declare no competing interests.
Figures
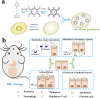
Fig. 1. Schematic illustration of the design of COF-based artificial probiotics for IBD therapy. (a) The synthesis of artificial probiotics. (b) The artificial probiotics for the treatment of IBD by modulating the gut microbiota, suppressing intestinal inflammation, protecting the intestinal epithelial cells, and regulating immunity .
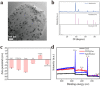
Fig. 2. Characterization of COF-based artificial probiotics and other related materials. (a) TEM image and (b) XRD pattern of the Aprobiotics. (c) Zeta-potential of the prepared COF, COF@Au, yeast, YS, YS-PEI and Aprobiotics. (d) XPS peak of COF, COF@Au and the Aprobiotics.
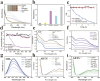
Fig. 3. The artificial probiotic exerted bioactivities in vitro (a and b) Measurement of gluconic acid product after Aprobiotics and glucose were incubated in PBS (pH = 7.4). (c) The variation in pH value of the Aprobiotic solution in the absence and presence of glucose in 0.5 mM PBS (pH = 7.4). (d) O2 concentration change of Aprobiotic solution upon the addition of glucose in PBS (pH = 7.4). (e) O2˙− scavenging activity of Au, COF, COF@Au, YS and Aprobiotics. (f) O2˙− scavenging activity of different concentrations of Aprobiotics. (g) OH˙ scavenging activity of Aprobiotics. (h) ABTS+˙ scavenging activity of COF, COF@Au, YS and Aprobiotics. (i) ABTS+˙ scavenging activity of different concentrations of Aprobiotics.
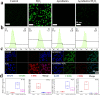
Fig. 4. Artificial probiotics suppressed excessive inflammatory response and reprogrammed the macrophage. (a) Fluorescence images and (b) flow cytometry analysis of ROS generation in RAW 264.7 cells treated with either H2O2 or Aprobiotics. (c) Immunofluorescent staining assay of macrophages after treatment with Aprobiotics for 1 day. (d) qPCR results of IL-10, TNF-α, Arg-1 and iNOS, respectively.
Fig. 5. Artificial probiotics exert outstanding efficacy in DSS-induced ulcerative colitis (UC) models: (a) the change in colon length of each group, (b) relative body weight change throughout the experiment, (c) H&E-staining assay of mice colon on day 12.
Fig. 6. Artificial probiotics modulated the gut microbiota and immune microenvironment. (a) Assessment of bacterial community richness (OUT). (b) PcoA analysis of the gut microbiome (c) Venn diagram of detected bacterial strains. (d) Relative abundance of the intestinal microbiome. (e) Heatmap of the relative abundance of bacterial taxa for each sample. (f) Circular plot representation of the interaction between genera of the gut microbial community of IBD mice after treatment with COF, COF@Au, and Aprobiotics. (g) Histological studies with immunohistochemical staining of mice colon on day 12.
Similar articles
- Current understanding of microbiota- and dietary-therapies for treating inflammatory bowel disease.
Eom T, Kim YS, Choi CH, Sadowsky MJ, Unno T. Eom T, et al. J Microbiol. 2018 Mar;56(3):189-198. doi: 10.1007/s12275-018-8049-8. Epub 2018 Feb 28. J Microbiol. 2018. PMID: 29492876 Review. - Regulation of gut microbiota-bile acids axis by probiotics in inflammatory bowel disease.
Li L, Liu T, Gu Y, Wang X, Xie R, Sun Y, Wang B, Cao H. Li L, et al. Front Immunol. 2022 Sep 23;13:974305. doi: 10.3389/fimmu.2022.974305. eCollection 2022. Front Immunol. 2022. PMID: 36211363 Free PMC article. Review. - Role of Lactic Acid Probiotic Bacteria in IBD.
Vemuri R, Gundamaraju R, Eri R. Vemuri R, et al. Curr Pharm Des. 2017;23(16):2352-2355. doi: 10.2174/1381612823666170207100025. Curr Pharm Des. 2017. PMID: 28176664 Review. - The Roles of Inflammation, Nutrient Availability and the Commensal Microbiota in Enteric Pathogen Infection.
Stecher B. Stecher B. Microbiol Spectr. 2015 Jun;3(3). doi: 10.1128/microbiolspec.MBP-0008-2014. Microbiol Spectr. 2015. PMID: 26185088 - Probiotic Cocktail Alleviates Intestinal Inflammation Through Improving Gut Microbiota and Metabolites in Colitis Mice.
Zhu Y, Xu Y, Wang X, Rao L, Yan X, Gao R, Shen T, Zhou Y, Kong C, Zhou L. Zhu Y, et al. Front Cell Infect Microbiol. 2022 Jun 15;12:886061. doi: 10.3389/fcimb.2022.886061. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35782138 Free PMC article.
Cited by
- Effect of probiotics intake on constipation in children: an umbrella review.
Dong M, Wu Y, Zhang M, Chen P, Zhang Z, Wang S. Dong M, et al. Front Nutr. 2023 Sep 1;10:1218909. doi: 10.3389/fnut.2023.1218909. eCollection 2023. Front Nutr. 2023. PMID: 37720380 Free PMC article. Review. - The Combination of Bacillus natto JLCC513 and Ginseng Soluble Dietary Fiber Attenuates Ulcerative Colitis by Modulating the LPS/TLR4/NF-κB Pathway and Gut Microbiota.
Ma M, Li Y, He Y, Li D, Niu H, Sun M, Miao X, Su Y, Zhang H, Hua M, Wang J. Ma M, et al. J Microbiol Biotechnol. 2024 Jun 28;34(6):1287-1298. doi: 10.4014/jmb.2402.02027. Epub 2024 May 10. J Microbiol Biotechnol. 2024. PMID: 38783703 Free PMC article. - Probiotics for inflammatory bowel disease: Is there sufficient evidence?
Ma Y, Yang D, Huang J, Liu K, Liu H, Wu H, Bao C. Ma Y, et al. Open Life Sci. 2024 Apr 5;19(1):20220821. doi: 10.1515/biol-2022-0821. eCollection 2024. Open Life Sci. 2024. PMID: 38585636 Free PMC article. Review. - Insights on Current Strategies to Decolonize the Gut from Multidrug-Resistant Bacteria: Pros and Cons.
Roson-Calero N, Ballesté-Delpierre C, Fernández J, Vila J. Roson-Calero N, et al. Antibiotics (Basel). 2023 Jun 19;12(6):1074. doi: 10.3390/antibiotics12061074. Antibiotics (Basel). 2023. PMID: 37370393 Free PMC article.
References
LinkOut - more resources
Full Text Sources