mRNA-From COVID-19 Treatment to Cancer Immunotherapy - PubMed (original) (raw)
Review
mRNA-From COVID-19 Treatment to Cancer Immunotherapy
Werner Krause. Biomedicines. 2023.
Abstract
This review provides an overview covering mRNA from its use in the COVID-19 pandemic to cancer immunotherapy, starting from the selection of appropriate antigens, tumor-associated and tumor-specific antigens, neoantigens, the basics of optimizing the mRNA molecule in terms of stability, efficacy, and tolerability, choosing the best formulation and the optimal route of administration, to summarizing current clinical trials of mRNA vaccines in tumor therapy.
Keywords: COVID-19; cancer vaccine; clinical applications; immunotherapy; mRNA; neoantigens; tumor antigens.
Conflict of interest statement
Krause is a retired employee of Bayer AG, Berlin, Germany.
Figures
Figure 1
(A): General structure of mRNA containing the four nucleobases adenine, cystine, guanine, and uracil, linked to ribose. The resulting nucleosides are connected via phosphate groups. The starting (5′ cap) position is formed by 7-methylguanosine with a three-phosphate moiety as a linker to the first nucleotide. At the end of the molecule, multiple adenosine moieties are attached. A codon comprises three nucleotides, for example, AUG, which is the code for methionine. (B): Functional structure of mRNA; details of functions are provided in Table 1. 5′ UTR: 5′ untranslated region. 3′ UTR: 3′ untranslated region. Poly(A)tail: multiple adenosine units.
Figure 2
Structure of saRNA containing an additional replicase section.
Figure 3
Major types of cancer immunotherapy with their benefits in green and their downsides in red.
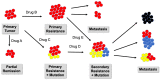
Figure 4
Development of cancer heterogeneity and formation of neoantigens. Treatment with a drug (A, B, C, D, or E) can result in cure, partial remission, or primary resistance that can progress to metastases and/or to mutation resulting in new cancer entities presenting neoantigens. Primary tumor:  . Tumor cells expressing neoantigens A
. Tumor cells expressing neoantigens A  , B
, B  , or C
, or C  .
.
Similar articles
- The Rapid Development and Early Success of Covid 19 Vaccines Have Raised Hopes for Accelerating the Cancer Treatment Mechanism.
Amanpour S. Amanpour S. Arch Razi Inst. 2021 Mar;76(1):1-6. doi: 10.22092/ari.2021.353761.1612. Epub 2021 Mar 1. Arch Razi Inst. 2021. PMID: 33818952 Free PMC article. - Identification of alternative splicing-derived cancer neoantigens for mRNA vaccine development.
Cheng R, Xu Z, Luo M, Wang P, Cao H, Jin X, Zhou W, Xiao L, Jiang Q. Cheng R, et al. Brief Bioinform. 2022 Mar 10;23(2):bbab553. doi: 10.1093/bib/bbab553. Brief Bioinform. 2022. PMID: 35279714 - Therapeutic Vaccines Targeting Neoantigens to Induce T-Cell Immunity against Cancers.
Pao SC, Chu MT, Hung SI. Pao SC, et al. Pharmaceutics. 2022 Apr 15;14(4):867. doi: 10.3390/pharmaceutics14040867. Pharmaceutics. 2022. PMID: 35456701 Free PMC article. Review. - Engineering neoantigen vaccines to improve cancer personalized immunotherapy.
Liu Z, Lv J, Dang Q, Liu L, Weng S, Wang L, Zhou Z, Kong Y, Li H, Han Y, Han X. Liu Z, et al. Int J Biol Sci. 2022 Sep 1;18(15):5607-5623. doi: 10.7150/ijbs.76281. eCollection 2022. Int J Biol Sci. 2022. PMID: 36263174 Free PMC article. Review. - Targeting neoantigens for cancer immunotherapy.
Zhao X, Pan X, Wang Y, Zhang Y. Zhao X, et al. Biomark Res. 2021 Jul 28;9(1):61. doi: 10.1186/s40364-021-00315-7. Biomark Res. 2021. PMID: 34321091 Free PMC article. Review.
Cited by
- Intradermal Delivery of Naked mRNA Vaccines via Iontophoresis.
Hasan M, Khatun A, Kogure K. Hasan M, et al. Pharmaceutics. 2023 Nov 26;15(12):2678. doi: 10.3390/pharmaceutics15122678. Pharmaceutics. 2023. PMID: 38140019 Free PMC article. Review. - The Role of Human Endogenous Retroviruses in Cancer Immunotherapy of the Post-COVID-19 World.
Logotheti S, Stiewe T, Georgakilas AG. Logotheti S, et al. Cancers (Basel). 2023 Nov 7;15(22):5321. doi: 10.3390/cancers15225321. Cancers (Basel). 2023. PMID: 38001581 Free PMC article. - Bibliometric analysis of RNA vaccines for cancer.
Yang X, Kang J, Xing Z, Sun Y, Liu Z, Li N, Niu J. Yang X, et al. Hum Vaccin Immunother. 2023 Aug 1;19(2):2231333. doi: 10.1080/21645515.2023.2231333. Epub 2023 Jul 18. Hum Vaccin Immunother. 2023. PMID: 37464256 Free PMC article.
References
- Corbett K.S., Edwards D.K., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R.A., Himansu S., Schäfer A., Ziwawo C.T., DiPiazza A.T., et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. - DOI - PMC - PubMed
Publication types
Grants and funding
This research received no external funding.
LinkOut - more resources
Full Text Sources