Spinal cord stimulation for low back pain - PubMed (original) (raw)
Review
Spinal cord stimulation for low back pain
Adrian C Traeger et al. Cochrane Database Syst Rev. 2023.
Abstract
Background: Spinal cord stimulation (SCS) is a surgical intervention used to treat persistent low back pain. SCS is thought to modulate pain by sending electrical signals via implanted electrodes into the spinal cord. The long term benefits and harms of SCS for people with low back pain are uncertain.
Objectives: To assess the effects, including benefits and harms, of SCS for people with low back pain.
Search methods: On 10 June 2022, we searched CENTRAL, MEDLINE, Embase, and one other database for published trials. We also searched three clinical trials registers for ongoing trials.
Selection criteria: We included all randomised controlled trials and cross-over trials comparing SCS with placebo or no treatment for low back pain. The primary comparison was SCS versus placebo, at the longest time point measured in the trials. Major outcomes were mean low back pain intensity, function, health-related quality of life, global assessment of efficacy, withdrawals due to adverse events, adverse events, and serious adverse events. Our primary time point was long-term follow-up (≥ 12 months).
Data collection and analysis: We used standard methodological procedures expected by Cochrane.
Main results: We included 13 studies with 699 participants: 55% of participants were female; mean age ranged from 47 to 59 years; and all participants had chronic low back pain with mean duration of symptoms ranging from five to 12 years. Ten cross-over trials compared SCS with placebo. Three parallel-group trials assessed the addition of SCS to medical management. Most studies were at risk of performance and detection bias from inadequate blinding and selective reporting bias. The placebo-controlled trials had other important biases, including lack of accounting for period and carryover effects. Two of the three parallel trials assessing SCS as an addition to medical management were at risk of attrition bias, and all three had substantial cross-over to the SCS group for time points beyond six months. In the parallel-group trials, we considered the lack of placebo control to be an important source of bias. None of our included studies evaluated the impact of SCS on mean low back pain intensity in the long term (≥ 12 months). The studies most often assessed outcomes in the immediate term (less than one month). At six months, the only available evidence was from a single cross-over trial (50 participants). There was moderate-certainty evidence that SCS probably does not improve back or leg pain, function, or quality of life compared with placebo. Pain was 61 points (on a 0- to 100-point scale, 0 = no pain) at six months with placebo, and 4 points better (8.2 points better to 0.2 points worse) with SCS. Function was 35.4 points (on a 0- to 100-point scale, 0 = no disability or best function) at six months with placebo, and 1.3 points better (3.9 points better to 1.3 points worse) with SCS. Health-related quality of life was 0.44 points out of 1 (0 to 1 index, 0 = worst quality of life) at six months with placebo, and 0.04 points better (0.16 points better to 0.08 points worse) with SCS. In that same study, nine participants (18%) experienced adverse events and four (8%) required revision surgery. Serious adverse events with SCS included infections, neurological damage, and lead migration requiring repeated surgery. We could not provide effect estimates of the relative risks as events were not reported for the placebo period. In parallel trials assessing SCS as an addition to medical management, it is uncertain whether, in the medium or long term, SCS can reduce low back pain, leg pain, or health-related quality of life, or if it increases the number of people reporting a 50% improvement or better, because the certainty of the evidence was very low. Low-certainty evidence suggests that adding SCS to medical management may slightly improve function and slightly reduce opioid use. In the medium term, mean function (0- to 100-point scale; lower is better) was 16.2 points better with the addition of SCS to medical management compared with medical management alone (95% confidence interval (CI) 19.4 points better to 13.0 points better; I2 = 95%; 3 studies, 430 participants; low-certainty evidence). The number of participants reporting opioid medicine use was 15% lower with the addition of SCS to medical management (95% CI 27% lower to 0% lower; I2 = 0%; 2 studies, 290 participants; low-certainty evidence). Adverse events with SCS were poorly reported but included infection and lead migration. One study found that, at 24 months, 13 of 42 people (31%) receiving SCS required revision surgery. It is uncertain to what extent the addition of SCS to medical management increases the risk of withdrawals due to adverse events, adverse events, or serious adverse events, because the certainty of the evidence was very low.
Authors' conclusions: Data in this review do not support the use of SCS to manage low back pain outside a clinical trial. Current evidence suggests SCS probably does not have sustained clinical benefits that would outweigh the costs and risks of this surgical intervention.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
AT: provided paid consultancy on models of physiotherapy care to a health service provider in 2017. SG: none known IAH: employed by New South Wales (NSW) Ministry of Health, University of NSW, and Australian Orthopaedic Association, and received royalties for a 2016 book, Surgery, the Ultimate Placebo, and a 2021 book, Hippocrasy, How Doctors Are Betraying Their Oath. CGM: has received competitive grants from government agencies and industry to support his research. As an invited speaker at conferences, he has had his expenses covered and also received small gifts, such as a box of chocolates or a bottle of wine. He has received honoraria for marking theses, reviewing grants, and preparing talks.
Figures
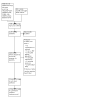
1
PRISMA study flow diagram
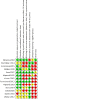
2
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
4
Comparison 1: spinal cord stimulation versus placebo. Outcome 1.1: low back pain intensity (0‐100) at immediate‐term follow‐up (< 1 month)
5
Comparison 1: spinal cord stimulation versus placebo. Outcome 1.3: leg pain intensity (0‐100) at immediate‐term follow‐up (< 1 month)
6
Comparison 2: spinal cord stimulation plus medical management versus medical management alone. Outcome 2.2: low back pain intensity at medium‐term follow‐up (≥ 3 months to < 12 months)
7
Comparison 2: spinal cord stimulation plus medical management versus medical management alone. Outcome 2.4: leg pain intensity at medium‐term follow‐up (≥ 3 months to < 12 months)
8
Comparison 2: spinal cord stimulation plus medical management versus medical management alone. Outcome 2.6: function at medium‐term follow‐up (≥ 3 months to < 12 months)
9
Comparison 2: spinal cord stimulation plus medical management versus medical management alone. Outcome 2.7: health‐related quality of life at medium‐term follow‐up (≥ 3 months to < 12 months)
10
Comparison 2: spinal cord stimulation plus medical management versus medical management alone. Outcome 2.8: global assessment of efficacy at medium‐term follow‐up (≥ 3 months to < 12 months)
1.1. Analysis
Comparison 1: Spinal cord stimulation (SCS) versus placebo, Outcome 1: Low back pain intensity (0‐100) at immediate‐term follow‐up (< 1 month)
1.2. Analysis
Comparison 1: Spinal cord stimulation (SCS) versus placebo, Outcome 2: Low back pain intensity (0‐100) at medium‐term follow‐up (≥ 3 mo to < 12 mo)
1.3. Analysis
Comparison 1: Spinal cord stimulation (SCS) versus placebo, Outcome 3: Leg pain intensity (0‐100) at immediate‐term follow‐up (< 1 month)
1.4. Analysis
Comparison 1: Spinal cord stimulation (SCS) versus placebo, Outcome 4: Leg pain intensity (0‐100) at medium‐term follow‐up (≥ 3 mo to < 12 mo)
1.5. Analysis
Comparison 1: Spinal cord stimulation (SCS) versus placebo, Outcome 5: Function (0‐100) at immediate‐term follow‐up (< 1 month)
1.6. Analysis
Comparison 1: Spinal cord stimulation (SCS) versus placebo, Outcome 6: Function (0‐100) at medium‐term follow‐up (≥ 3 mo to < 12 mo)
1.7. Analysis
Comparison 1: Spinal cord stimulation (SCS) versus placebo, Outcome 7: Health‐related quality of life (0‐1 index) at immediate‐term follow‐up (< 1 month)
1.8. Analysis
Comparison 1: Spinal cord stimulation (SCS) versus placebo, Outcome 8: Health‐related quality of life (0‐1 index) at medium‐term follow‐up (≥ 3 mo to < 12 mo)
2.1. Analysis
Comparison 2: Spinal cord stimulation (SCS) plus medical management versus medical management alone, Outcome 1: Low back pain intensity (0‐100) at short‐term follow‐up (≥ 1 mo to < 3 mo)
2.2. Analysis
Comparison 2: Spinal cord stimulation (SCS) plus medical management versus medical management alone, Outcome 2: Low back pain intensity (0‐100) at medium‐term follow‐up (≥ 3 mo to < 12 mo)
2.3. Analysis
Comparison 2: Spinal cord stimulation (SCS) plus medical management versus medical management alone, Outcome 3: Leg pain intensity (0‐100) at short‐term follow‐up (≥ 1 mo to < 3 mo)
2.4. Analysis
Comparison 2: Spinal cord stimulation (SCS) plus medical management versus medical management alone, Outcome 4: Leg pain intensity (0‐100) at medium‐term follow‐up (≥ 3 mo to < 12 mo)
2.5. Analysis
Comparison 2: Spinal cord stimulation (SCS) plus medical management versus medical management alone, Outcome 5: Function (0‐100) at short‐term follow‐up (≥ 1 mo to < 3 mo)
2.6. Analysis
Comparison 2: Spinal cord stimulation (SCS) plus medical management versus medical management alone, Outcome 6: Function (0‐100) at medium‐term follow‐up (≥ 3 mo to < 12 mo)
2.7. Analysis
Comparison 2: Spinal cord stimulation (SCS) plus medical management versus medical management alone, Outcome 7: Health‐related quality of life (0‐100) at medium‐term follow‐up (≥ 3 mo to < 12 mo)
2.8. Analysis
Comparison 2: Spinal cord stimulation (SCS) plus medical management versus medical management alone, Outcome 8: Global assessment of efficacy at medium‐term follow‐up (≥ 3 mo to < 12 mo)
2.9. Analysis
Comparison 2: Spinal cord stimulation (SCS) plus medical management versus medical management alone, Outcome 9: Global assessment of efficacy at long‐term follow‐up (≥ 12 mo)
2.10. Analysis
Comparison 2: Spinal cord stimulation (SCS) plus medical management versus medical management alone, Outcome 10: Withdrawals due to adverse events at longest follow‐up
2.11. Analysis
Comparison 2: Spinal cord stimulation (SCS) plus medical management versus medical management alone, Outcome 11: Proportion with any adverse event at longest follow‐up
2.12. Analysis
Comparison 2: Spinal cord stimulation (SCS) plus medical management versus medical management alone, Outcome 12: Proportion with serious adverse event at longest follow‐up
2.13. Analysis
Comparison 2: Spinal cord stimulation (SCS) plus medical management versus medical management alone, Outcome 13: Medication use 1 (number (%) taking opioid medicines) at medium‐term follow‐up (≥ 3 mo to < 12 mo)
2.14. Analysis
Comparison 2: Spinal cord stimulation (SCS) plus medical management versus medical management alone, Outcome 14: Medication use 2 (daily MME) at medium‐term follow‐up (≥ 3 mo to < 12 mo)
2.15. Analysis
Comparison 2: Spinal cord stimulation (SCS) plus medical management versus medical management alone, Outcome 15: Work status 1 (number returned to work) at medium‐term follow‐up (≥ 3 mo to < 12 mo)
Comment in
- Striking errors in the methodology, execution, and conclusions of the Cochrane Library review of spinal cord stimulation for low back pain by Traeger et al.
Durbhakula S, Broachwala MY, Schuster NM, McCormick ZL. Durbhakula S, et al. Pain Med. 2023 Aug 1;24(8):923-925. doi: 10.1093/pm/pnad047. Pain Med. 2023. PMID: 37067491 Free PMC article. No abstract available. - A comprehensive response to the letter of Traeger and colleagues on spinal cord stimulation for low back pain.
Durbhakula S, Broachwala MY, Schuster NM, McCormick ZL. Durbhakula S, et al. Pain Med. 2023 Sep 1;24(9):1129-1130. doi: 10.1093/pm/pnad060. Pain Med. 2023. PMID: 37195450 No abstract available. - Spinal Cord Stimulation for Low Back Pain.
Nelson B, Haynie D, Leggit JC. Nelson B, et al. Am Fam Physician. 2023 Dec;108(6):542-543. Am Fam Physician. 2023. PMID: 38215413 No abstract available.
Comment on
- Response to Durbhakula and colleagues.
Traeger AC, Gilbert SE, Harris IA, Maher CG. Traeger AC, et al. Pain Med. 2023 Sep 1;24(9):1127-1128. doi: 10.1093/pm/pnad061. Pain Med. 2023. PMID: 37195461 No abstract available.
Similar articles
- Implanted spinal neuromodulation interventions for chronic pain in adults.
O'Connell NE, Ferraro MC, Gibson W, Rice AS, Vase L, Coyle D, Eccleston C. O'Connell NE, et al. Cochrane Database Syst Rev. 2021 Dec 2;12(12):CD013756. doi: 10.1002/14651858.CD013756.pub2. Cochrane Database Syst Rev. 2021. PMID: 34854473 Free PMC article. Review. - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - Arthroscopic surgery for degenerative knee disease (osteoarthritis including degenerative meniscal tears).
O'Connor D, Johnston RV, Brignardello-Petersen R, Poolman RW, Cyril S, Vandvik PO, Buchbinder R. O'Connor D, et al. Cochrane Database Syst Rev. 2022 Mar 3;3(3):CD014328. doi: 10.1002/14651858.CD014328. Cochrane Database Syst Rev. 2022. PMID: 35238404 Free PMC article. Review. - Subacromial decompression surgery for rotator cuff disease.
Karjalainen TV, Jain NB, Page CM, Lähdeoja TA, Johnston RV, Salamh P, Kavaja L, Ardern CL, Agarwal A, Vandvik PO, Buchbinder R. Karjalainen TV, et al. Cochrane Database Syst Rev. 2019 Jan 17;1(1):CD005619. doi: 10.1002/14651858.CD005619.pub3. Cochrane Database Syst Rev. 2019. PMID: 30707445 Free PMC article. - Autologous blood and platelet-rich plasma injection therapy for lateral elbow pain.
Karjalainen TV, Silagy M, O'Bryan E, Johnston RV, Cyril S, Buchbinder R. Karjalainen TV, et al. Cochrane Database Syst Rev. 2021 Sep 30;9(9):CD010951. doi: 10.1002/14651858.CD010951.pub2. Cochrane Database Syst Rev. 2021. PMID: 34590307 Free PMC article. Review.
Cited by
- MicroRNA-29a Mitigates Laminectomy-Induced Spinal Epidural Fibrosis and Gait Dysregulation by Repressing TGF-β1 and IL-6.
Lin IT, Lin YH, Lian WS, Wang FS, Wu RW. Lin IT, et al. Int J Mol Sci. 2023 May 23;24(11):9158. doi: 10.3390/ijms24119158. Int J Mol Sci. 2023. PMID: 37298111 Free PMC article. - Evolution of treatment for unspecific back pain: From past to future.
Herrera D, Hartard C, Ben Saad H, Montanari Mota L, Alves Dos Santos V, Sinha C, Jedidi R, Hartard D, Khaled S, Hartard S, Hartard M. Herrera D, et al. Tunis Med. 2024 Sep 5;102(9):509-512. doi: 10.62438/tunismed.v102i9.5162. Tunis Med. 2024. PMID: 39287341 Free PMC article. Review. English. - Failed back surgery syndrome-terminology, etiology, prevention, evaluation, and management: a narrative review.
Yeo J. Yeo J. J Yeungnam Med Sci. 2024 Jul;41(3):166-178. doi: 10.12701/jyms.2024.00339. Epub 2024 Jun 10. J Yeungnam Med Sci. 2024. PMID: 38853538 Free PMC article. - Spinal cord stimulation for chronic pain treatment following sacral chordoma resection: illustrative case.
Taghlabi KM, Hassan T, Somawardana IA, Rajendran S, Doomi A, Bhenderu LS, Cruz-Garza JG, Faraji AH. Taghlabi KM, et al. J Neurosurg Case Lessons. 2023 Dec 25;6(26):CASE23540. doi: 10.3171/CASE23540. Print 2023 Dec 25. J Neurosurg Case Lessons. 2023. PMID: 38145561 Free PMC article.
References
References to studies included in this review
Al‐Kaisy 2018 {published data only}
- Al-Kaisy A, Palmisani S, Pang D, Sanderson K, Wesley S, Tan Y, et al. Prospective, randomised, sham-control, double blind, crossover trail of subthreshold spinal cord stimulation at various kilohertz frequencies in subjects suffering from failed back surgery syndrome (SCS Frequency Study). Neuromodulation 2018;21(5):457-65. - PubMed
- Al-Kaisy A, Palmisani S, Sanderson KK, Tan Y, McCammon S. Subject therapy preference post randomized phase in a spinal cord stimulation study using higher frequencies. Neuromodulation 2018;20(7):e232.
- NCT01750229. Randomized controlled double-blind cross-over trial evaluating the role of frequencies on spinal cord stimulation in the management of failed back surgery syndrome (SCS Frequency Study). clinicaltrials.gov/ct2/show/NCT01750229 (first posted 17 December 2012).
De Ridder 2013 {published data only}
- De Ridder D, Plazie M, Kamerling, N, Menovsky, Vanneste S. Burst spinal cord stimulation for limb and back pain. World Neurosurgery 2013;80(5):642-49.e1. - PubMed
- De Ridder D, Vanneste S. Burst and tonic spinal cord stimulation: different and common brain mechanisms. Neuromodulation 2015;19(1):47-59. - PubMed
Eisenberg 2015 {published data only}
- Eisenberg E, Burstein Y, Suzan E, Treister R, Aviram J. Spinal cord stimulation attenuates temporal summation in patients with neuropathic pain. Pain 2015;156(3):381-85. - PubMed
Eldabe 2020 {published data only}
- Eldabe S, Duarte R, Gulve A, Williams H, Garner F, Brookes M, et al. Analgesic efficacy of "burst" and tonic (500 Hz) spinal cord stimulation patterns: a randomized placebo-controlled crossover study. Neuromodulation 2020;24(3):471-78. - PubMed
Hara 2022 {published data only}
- Hara S, Andresen H, Solheim O, Carlsen SM, Sundstrøm T, Lønne G, et al. Effect of spinal cord burst stimulation vs placebo stimulation on disability in patients with chronic radicular pain after lumbar spine surgery: a randomized clinical trial. JAMA 2022;328(15):1506-14. [DOI: 10.1001/jama.2022.18231] - DOI - PMC - PubMed
- NCT03546738. Spinal cord burst stimulation for chronic radicular pain following lumbar spine surgery. clinicaltrials.gov/ct2/show/NCT03546738 (first posted 6 June 2018).
Kapural 2022 {published and unpublished data}
- ISRCTN87648175. Comparison of HF10 therapy combined with Conventional Medical Managment (CMM) to CMM alone in the treatment of chronic back pain. www.isrctn.com/ISRCTN87648175 (first posted 21 November 2017).
- Kapural L, Jameson J, Johnson C, Kloster D, Calodney A, Kosek P, et al. Treatment of nonsurgical refractory back pain with high-frequency spinal cord stimulation at 10 kHz: 12-month results of a pragmatic, multicenter, randomized controlled trial. Journal of Neurosurgery 2022;37(2):188-99. [DOI: 10.3171/2021.12.SPINE211301] - DOI - PubMed
- Patel N, Calodney A, Kapural L, Province-Azalde R, Lad SP, Pilitsis J, et al. High-frequency spinal cord stimulation at 10 kHz for the treatment of nonsurgical refractory back pain: design of a pragmatic, multicenter, randomized controlled trial. Pain Practice 2021;21(2):171-83. [DOI: 10.1111/papr.12945] - DOI - PMC - PubMed
- Province-Azalde R, Patel NP, Wu C, Pilitsis J, Lad N, Calodney AK, et al. Taking spinal cord stimulation beyond failed back surgery syndrome: design of a multicenter RCT. Neuromodulation 2019;22(3):E286.
Kumar 2007 {published data only}
- Eldabe S, Buchser E, Kumar K, Taylor R. Function and quality of life in failed back surgery syndrome patients following spinal cord stimulation and conventional medical management. European Journal of Pain 2009;13:S45. - PubMed
- Eldabe S, Buchser E, Kumar K, Taylor R. Pain in failed back surgery syndrome patients following spinal cord stimulation and conventional medical management. European Journal of Pain 2009;13:S135. - PubMed
- Eldabe S, Kumar K, Buchser E, Taylor RS. An analysis of the components of pain, function, and health-related quality of life in patients with failed back surgery syndrome treated with spinal cord stimulation or conventional medical management. Neuromodulation 2010;13(3):201-09. [DOI: 10.1111/j.1525-1403.2009.00271.x] - DOI - PubMed
- ISRCTN77527324. A prospective, randomised, controlled, multicentre study to evaluate the effectiveness and cost-effectiveness of spinal cord stimulation using the Synergy System in reducing pain in patients with failed back surgery syndrome compared to conventional medical management (PROCESS study). isrctn.com/ISRCTN77527324 (first posted 1 July 2003). [DOI: 10.1186/ISRCTN77527324] - DOI
- Jacques L. Poster 60: Spinal cord stimulation versus conventional medical management: long-term results from the PROCESS Study: a multi-center randomized controlled trial of patients with failed back surgery syndrome. Archives of Physical Medicine and Rehabilitation 2008;89:E38.
Perruchoud 2013 {published data only}
Rigoard 2019 {published data only}
- NCT01697358. Prospective, randomized study of multicolumn implantable lead stimulation for predominant low back pain. clinicaltrials.gov/ct2/show/NCT01697358 (first posted 2 October 2012).
- North RB, Deruytter MM, Vangeneugden JJ, Raftopoulos C, Van Havenbergh T, Desai MJ, et al. Perioperative infections and prolonged SCS trial duration (PROMISE study). Neuromodulation 2018;21(3):e11.
- Rigoard P, Desai M, North R, Taylor R, Annemans L, Greening, C, et al. Spinal cord stimulation for predominant low back pain in failed back surgery syndrome: design and enrollment of an international multicenter randomized controlled trial (PROMISE Study). Neuromodulation 2013;16(5):e74. - PMC - PubMed
Schu 2014 {published data only}
- Schu S, Bara G, Vesper J. Burst or tonic stimulation' first results of a placebo controlled, doubled blinded, randomized study for the treatment of FBSS patients. Pain Practice 2014;14(68):68-undefined.
- Schu S, Slotty PJ, Bara G, Knop M, Edgar D, Vesper J. A prospective, randomised, double-blind, placebo-controlled study to examine the effectiveness of burst spinal cord stimulation patterns for the treatment of failed back surgery syndrome. Neuromodulation 2014;17(5):443-50. [DOI: 10.1111/ner.12197] - DOI - PubMed
- Vesper J, Gregor B, Slotty P. Burst or tonic stimulation? Results of a placebo controlled, double blinded, randomized study for the treatment of FBSS patients - 2 year follow-up. Pain Practice 2016;16(S1):74-75.
- Vesper J, Slotty PJ, Schu S. Burst or tonic stimulation? Results of a placebo controlled, double blinded, randomized study for the treatment of FBSS patients - 3 year follow-up. Neuromodulation 2017;20(7):e170.
Sokal 2020 {published data only}
- NCT03957395. Comparison of effectiveness of tonic, high frequency and burst spinal cord stimulation in chronic pain syndromes. clinicaltrials.gov/show/NCT03957395 (first posted 21 May 2019).
- Sokal P, Malukiewicz A, Kieronska S, Murawska J, Guzowski C, Rudas M, et al. Sub-perception and supra-perception spinal cord stimulation in chronic pain syndrome: a randomized, semi-double-blind, crossover, placebo-controlled trial. Journal of Clinical Medicine 2020;9(9):1-15. [DOI: 10.3390/jcm9092810] - DOI - PMC - PubMed
Sweet 2016 {published data only}
- NCT05283863. Direct comparison of spinal cord stimulator parameter settings. clinicaltrials.gov/ct2/show/NCT05283863 (first posted 17 March 2022).
Wolter 2012 {published data only}
References to studies excluded from this review
ACTRN12614000236695 {published data only}
- ACTRN12614000236695. HFS ONE: is high frequency spinal cord stimulation more effective than sham treatment during a 20 day trial period for lumbar spine pain and leg pain? A randomised double-blinded placebo-controlled cross over trial. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365853 (first posted 5 March 2014).
ACTRN12617001541392 {published data only}
- ACTRN12617001541392. Assessing the outcomes of patients who undergo spinal cord stimulation compared to patients who undergo spinal fusion surgery for the treatment of chronic low back pain. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373866 (first posted 7 November 2017).
Amirdelfan 2018 {published data only}
- Amirdelfan KT, Shrivalkar B, Reddy P, Reddy S, Khanna V, Kramer K, et al. Non-invasive high-frequency impulse neuromodulation for treatment of chronic back pain: a multicenter, randomized, sham-controlled trial. Neuromodulation 2019;22(3):E294.
Andersen 2009 {published data only}
- Andersen T, Christensen FB, Egund N, Ernst C, Fruensgaard S, Ostergaard J, et al. The effect of electrical stimulation on lumbar spinal fusion in older patients: a randomized, controlled, multi-center trial. Part 2: fusion rates. Spine 2009;34(21):2241-7. - PubMed
Baranidharan 2020 {published data only}
- Baranidharan GF, Bretherton R, Crowther B, Cooper T, Castino L, Radford H. One‐year results of prospective research study using 10 kHz spinal cord stimulation in persistent nonoperated low back pain of neuropathic origin: Maiden Back Study. Neuromodulation 2021;24(3):479-87. - PubMed
Billot 2020 {published data only}
- Billot M, Naiditch N, Brandet C, Lorgeoux B, Baron S, Ounajim A, et al. Comparison of conventional, burst and high-frequency spinal cord stimulation on pain relief in refractory failed back surgery syndrome patients: study protocol for a prospective randomized double-blinded cross-over trial (MULTIWAVE study). Trials 2020;21(696):undefined. [DOI: 10.1186/s13063-020-04587-6] - DOI - PMC - PubMed
De Andres 2017 {published data only}
- De Andres J, Monsalve-Dolz V, Fabregat-Cid G, Villanueva-Perez V, Harutyunyan A, Asensio-Samper JM, et al. Prospective, randomized blind effect-on-outcome study of conventional vs high-frequency spinal cord stimulation in patients with pain and disability due to Failed Back Surgery Syndrome. Pain Medicine 2017;18:2401-21. - PubMed
Deer 2014 {published data only}
- Deer T, Levy R, Grigsby E, Poree L, Staats P, Caraway D, et al. A prospective, randomized, multi-center, controlled clinical trial to assess the safety and efficacy of the spinal modulation Axium neurostimulation system in the treatment of chronic pain (Accurate Trial): trial design. Neuromodulation 2014;18(2):e99-undefined.
Deer 2015b {published data only}
Eldabe 2020b {published data only}
- Eldabe S, Duarte RV, Gulve A, Thomson S, Baranidharan G, Houten R, et al. Does a screening trial for spinal cord stimulation in patients with chronic pain of neuropathic origin have clinical utility and cost-effectiveness (TRIAL-STIM)? A randomised controlled trial. Pain 2020;161(12):2820-9. - PMC - PubMed
ISRCTN13607429 2016 {published data only}
- ISRCTN13607429. Pain perception evaluation with paresthesia independent spinal cord stimulation therapy. www.isrctn.com/ISRCTN13607429 (first posted 27 July 2016).
Kapural 2015 {published data only}
- Kapural L, Yu C, Doust MW, Gliner BE, Vallejo R, Todd Sitzman B, et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain. Anesthesiology 2015;123:851-60. - PubMed
Kriek 2017 {published data only}
- Kriek N, Groeneweg JG, Stronks DL, de Ridder D, Huygen PM. Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: a multicentre, double-blind, randomized and placebo-controlled crossover trial. European Journal of Pain 2017;21:507-19. - PubMed
MacIver 2010 {published data only}
- Maclver K, Harmon D, Nurmikko T. The effect of spinal cord stimulation (SCS) on sensory changes in neuropathic pain. European Journal of Pain Supplements 2010;4:47-146.
Meier 2015 {published data only}
- Meier K, Nikolajsen L, Sorensen JC, Jensen TS. Effect of spinal cord stimulation on sensory characteristics: a randomized, blinded crossover study. Clinical Journal of Pain 2015;31:384-92. - PubMed
Mekhail 2020 {published data only}
- Mekhail N, Levy RM, Deer TR, Kapural L, Li S, Amirdelfan K, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurology 2020;19:123-34. - PubMed
NCT00200122 {published data only}https://clinicaltrials.gov/ct2/show/NCT00200122
- NCT00200122. Restore claims characterization study. clinicaltrials.gov/ct2/show/NCT00200122 (first posted 20 September 2005).
NCT01550562 2012 {published data only}
- NCT01550562. Effects of programming parameters on back pain relief in subthreshold spinal cord stimulation. clinicaltrials.gov/ct2/show/NCT01550562 (first posted 12 March 2012).
NCT02837822 {published data only}https://clinicaltrials.gov/ct2/show/NCT02837822
- NCT02837822. Effects of spinal cord stimulation on sensory perceptions of chronic pain patients. clinicaltrials.gov/ct2/show/NCT02837822 (first posted 20 July 2016).
NCT03312010 {published data only}
- NCT03312010. Tsunami DRG high frequency stimulation study. clinicaltrials.gov/ct2/show/NCT03312010 (first posted 17 October 2017).
North 1994 {published data only}
- North RB, Kidd DH, Lee MS, Piantodosi S. A prospective, randomized study of spinal cord stimulation versus reoperation for failed back surgery syndrome: initial results. Stereotactic and Functional Neurosurgery 1994;62(1-4):267-72. - PubMed
North 2005 {published data only}
- North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery 2005;56(1):98-106. - PubMed
North 2020 {published data only}
Roulaud 2015 {published data only}
- Roulaud M, Durand-Zaleski I, Ingrand P, Serrie A, Diallo B, Peruzzi P, et al. Multicolumn spinal cord stimulation for significant low back pain in failed back surgery syndrome: design of a national, multicentre, randomized, controlled health economics trial (ESTIMET Study). Neurochiurgie 2015;61(Suppl 1):S109-16. - PubMed
Thomson 2017 {published data only}
Tjepkema‐Cloostermans 2016 {published data only}
- Tjepkema-Cloostermans MC, Vos CC, Wolters R, Dijkstra-Scholten C, Lenders MW. Effect of burst stimulation evaluated in patients familiar with spinal cord stimulation. Neuromodulation 2016;19(5):492-7. - PubMed
Veizi 2017 {published data only}
- Veizi E, Hayek SM, North J, Brent Chafin T, Yearwood TL, Raso L, et al. Spinal cord stimulation (SCS) with anatomically guided (3D) neural targeting shows superior chronic axial low back pain relief compared to traditional SCS—LUMINA Study. Pain Medicine 2017;18:1534-48. - PubMed
Vesper 2017 {published data only}
- Vesper J, Slotty PJ, Poeggel-Kraemer KP, Littges H, Agnesi F, Venkatesan L. Therapeutic efficacy of Burst DR microdosing in treatment of chronic pain. Neuromodulation 2017;22(2):190-3. - PubMed
Vesper 2019 {published data only}
- Vesper J, Slotty P, Schu S, Poeggel-Kraemer K, Littges H, Van Looy P, et al. Burst SCS microdosing is as efficacious as standard burst SCS in treating chronic back and leg pain: results from a randomized controlled trial. Neuromodulation 2019;22:190-93. - PubMed
References to studies awaiting assessment
Mekel‐Bobrov 2017 {published data only}
- Mekel-Bobrov N, Rauck R, Maoz U. Differential mechanisms of action between paresthesia and paresthesia-free SCS: a PET study. Neuromodulation 2017;20:54. [DOI: 10.1111/ner.12572] - DOI
Miller 2015 {published data only}
- Miller NS, Vallejo R, Binyamin R, Lin S, Mekel-Bobrov N. Optimization of stimulation location is essential to sub-perception SCS: results of a double-blind randomized placebo-controlled trial. Neuromodulation 2015;18:e13–e106. [DOI: 10.1111/ner.12277] - DOI
Miller 2016 {published data only}
References to ongoing studies
ACTRN12620000720910 {published data only}
- ACTRN12620000720910. An evaluation of spinal cord stimulation for the treatment of chronic pain, also its effect on mood, sleep, physical activity and analgesic medicine requirements. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=379835&isReview=true (first registered 2 July 2020).
Ahmadi 2021 {published data only}
- Ahmadi R, Campos B, Hajiabadi MM, Doerr-Harim C, Tenckhoff S, Rasche D, et al. Efficacy of different spinal cord stimulation paradigms for the treatment of chronic neuropathic pain (PARS-trial): study protocol for a double-blinded, randomized, and placebo-controlled crossover trial. Trials 2021;22(1):87. [DOI: 10.1186/s13063-020-05013-7] - DOI - PMC - PubMed
Al‐Kaisy 2020 {published data only}
- Al-Kaisy A, Royds J, Palmisani S, Pang D, Wesley S, Taylor RS, et al. Multicentre, double-blind, randomised, sham-controlled trial of 10 khz high-frequency spinal cord stimulation for chronic neuropathic low back pain (MODULATE-LBP): a trial protocol. Trials 2020;21(1):111. [DOI: 10.1186/s13063-019-3831-4] [URL: www.ncbi.nlm.nih.gov/pmc/articles/PMC6986091/pdf/13063_2019_Article_3831...] - DOI - PMC - PubMed
ISRCTN10663814 {published data only}
- ISRCTN10663814. Comparison of spinal cord stimulation in combination with standard pain treatment versus standard pain treatment only in patients with intractable chronic back pain without previous history of spine surgery. www.isrctn.com/ISRCTN10663814 (first posted 29 June 2020).
ISRCTN33292457 {published data only}
- ISRCTN33292457. Senza spinal cord stimulation system for the treatment of chronic back and leg pain in failed back surgery syndrome (FBSS) patients. www.isrctn.com/ISRCTN33292457 (first registered 08 April 2011). [DOI: 10.1186/ISRCTN33292457] - DOI
NCT03419312 {published data only}
- NCT03419312. PET patterns, biomarkers and outcome in burst SCS treated FBSS patients (PET-SCS). clinicaltrials.gov/ct2/show/NCT03419312 (first posted 1 February 2018).
NCT03462147 {published data only}
- NCT03462147. Efficacy of spinal cord stimulation in patients with a failed back surgery syndrome. clinicaltrials.gov/ct2/show/NCT03462147 (first posted 12 March 2018).
NCT03718325 {published data only}https://clinicaltrials.gov/show/NCT03718325
- NCT03718325. Burst spinal cord stimulation (Burst-SCS) study. clinicaltrials.gov/ct2/show/NCT03718325 (first posted 24 October 2018).
NCT03858790 {published data only}
- NCT03858790. Efficacy and safety of spinal cord stimulation in patients with chronic intractable pain. clinicaltrials.gov/ct2/show/NCT03858790 (first posted 1 March 2019).
NCT04479787 {published data only}
- NCT04479787. Spinal cord stimulation vs. medical management for low back pain (DISTINCT). clinicaltrials.gov/ct2/show/NCT04479787 (first posted 21 July 2020).
NCT04676022 {published data only}
- NCT04676022. SCS as an option for chronic low back and/or leg pain instead of surgery (SOLIS). clinicaltrials.gov/ct2/show/NCT04676022 (first posted 19 December 2020).
NCT04732325 {published data only}
- NCT04732325. Sensory testing of multiple forms of spinal cord stimulation for pain. clinicaltrials.gov/ct2/show/NCT04732325 (first posted 1 February 2021).
Reiter 2019 {published data only}
- Reiters P, Eldridge P, Shiban E, Crossman J, Fritz AK, Lehmberg J, et al. High frequency spinal cord stimulation (HFSCS) at 10 kHz plus conventional medical management (CMM) versus conventional medical management alone for the treatment of non-surgical back pain. Neuromodulation 2019;22:e8. [URL: www.cochranelibrary.com/es/central/doi/10.1002/central/CN-02130554/refer...]
Additional references
Chiarotto 2018
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed prior to 21 February 2023. Available at covidence.org.
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Dionne 2008
- Dionne CE, Dunn KM, Croft PR, Nachemson AL, Buchbinder R, Walker BF, et al. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine 2008;33(1):95-103. - PubMed
Duarte 2020
Global Burden of Disease Study 2018
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study. Lancet 2018;392(10159):1789-858. - PMC - PubMed
Grider 2016
- Grider JS, Manchikanti L, Carayannopoulos A, Sharma ML, Balog CC, Harned ME, et al. Effectiveness of spinal cord stimulation in chronic spinal pain: a systematic review. Pain Physician 2016;19(1):E33-54. - PubMed
Hartvigsen 2018
- Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, et al. What low back pain is and why we need to pay attention. Lancet 2018;18(3):E333-46. - PubMed
Higgins 2011
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook-5-1.cochrane.org.
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Higgins 2021a
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Higgins 2021b
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Higgins 2021c
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Hoffmann 2014
Jones 2022
- Jones CM, Abdel Shaheed C, Ferreira G, Mannix L, Harris I, Buchbinder R, et al. Spinal cord stimulators: an analysis of the adverse events reported to the Australian Therapeutic Goods Administration. Journal of Patient Safety 2022;18(5):507-11. [DOI: doi: 10.1097/PTS.0000000000000971] - PMC - PubMed
Kemler 2000
- Kemler MA, Barendse GA, Kleef M, Vet HC, Rijks CP, Furnee CA, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. New England Journal of Medicine 2000;343(9):618-24. - PubMed
Mailis‐Gagnon 2013
Melzack 1965
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science (New York, NY) 1965;150(3699):971-9. - PubMed
Meyerson 2000
- Meyerson BA, Linderoth B. Mechanisms of spinal cord stimulation in neuropathic pain. Neurological Research 2000;22(3):285-92. - PubMed
NICE 2020
- National Institute for Health and Care Excellence. Neuropathic pain in adults: pharmacological management in non-specialist settings. Clinical guideline [CG173]; November 2013; updated September 2020. www.nice.org.uk/guidance/cg173 (accessed prior to 21 February 2023).
Nijs 2015
- Nijs J, Apeldoorn A, Hallegraeff H, Clark J, Smeets R, Malfliet A, et al. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician 2015;18(3):E333-46. - PubMed
O'Connell 2020
O'Connell 2021
Page 2021
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
PlotDigitizer [Computer program]
- PlotDigitizer. Available at: automeris.io/WebPlotDigitizer, 2020.
PRISMA Group 2009
PRWeb 2015
- PRWeb. Boston Scientific overtakes Medtronic in medical device market for back pain and Failed Back Surgery Syndrome; 2015. Available at: www.prweb.com/releases/2015/02/prweb12497221.htm.
RevMan Web 2020 [Computer program]
- Review Manager Web (RevMan Web). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Roussel 2013
- Roussel NA, Nijs J, Meeus M, Mylius V, Fayt C, Oostendorp R. Central sensitization and altered central pain processing in chronic low back pain: fact or myth? Clinical Journal of Pain 2013;29(7):625-38. - PubMed
Rubinstein 2012
Schünemann 2021a
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Schünemann 2021b
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Shamji 2015
- Shamji MF, Westwick HJ, Heary RF. Complications related to the use of spinal cord stimulation for managing persistent postoperative neuropathic pain after lumbar spinal surgery. Neurosurgical Focus 2015;39(4):E15. - PubMed
Thomson 2013
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources