A cell that dies during wild-type C. elegans development can function as a neuron in a ced-3 mutant - PubMed (original) (raw)
A cell that dies during wild-type C. elegans development can function as a neuron in a ced-3 mutant
L Avery et al. Cell. 1987.
Abstract
Mutations in the C. elegans gene ced-3 prevent almost all programmed cell deaths, so that in a ced-3 mutant there are many extra cells. We show that the pharyngeal neuron M4 is essential for feeding in wild-type worms, but in a ced-3 mutant, one of the extra cells, probably MSpaaaaap (the sister of M4), can sometimes take over M4's function. The function of MSpaaaaap, unlike that of M4, is variable and subnormal. One possible explanation is that its fate, being hidden by death and not subject to selection, has drifted randomly during evolution. We suggest that such cells may play roles in the evolution of cell lineage analogous to those played by pseudogenes in the evolution of genomes.
Figures
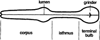
Figure 1. Anatomy of the Pharynx
Anterior is to the left.
Figure 2. Comparison of Intact Pharynx with Pharynx Lacking M4
(a) Nomarski differential interference contrast micrograph of the pharynx of an intact wild-type worm. (b) Tracing of (a). Anterior is left, dorsal up. Wild-type eggs were placed on an NGM plate that had been spread with ink. After 4–5 hr, the animals were mounted in sodium azide, which anesthetizes them, and photographed. The line down the center of the pharynx is the lumen, which is closed in normal anesthetized worms. The ink eaten by intact worms like this one is passed back to the intestine (not shown). (c) Nomarski photomicrograph of the pharynx of a wild-type worm in which M4 had been killed. (d) Tracing of(c). The worm was placed on an ink plate immediately after M4 was killed, and photographed 4–5 hr later under anesthesia. The lumen of the front half of the pharynx, including the entire corpus and the front half of the isthmus, is stuffed with ink. Scale bar = 10 µm.
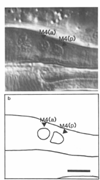
Figure 3. Two Nuclei at the M4 Position in ced-3
(a) Nomarski photograph of the posterior metacarpus and anterior isthmus of a ced-3 worm, showing two nuclei where wild-type has only M4. (b) Tracing of (a). Anterior left, dorsal up. Scale bar = 5 µm.
Similar articles
- Genetic control of programmed cell death in the nematode C. elegans.
Ellis HM, Horvitz HR. Ellis HM, et al. Cell. 1986 Mar 28;44(6):817-29. doi: 10.1016/0092-8674(86)90004-8. Cell. 1986. PMID: 3955651 - The Caenorhabditis elegans genes ced-3 and ced-4 act cell autonomously to cause programmed cell death.
Yuan JY, Horvitz HR. Yuan JY, et al. Dev Biol. 1990 Mar;138(1):33-41. doi: 10.1016/0012-1606(90)90174-h. Dev Biol. 1990. PMID: 2307287 - Mutations that affect neural cell lineages and cell fates during the development of the nematode Caenorhabditis elegans.
Horvitz HR, Sternberg PW, Greenwald IS, Fixsen W, Ellis HM. Horvitz HR, et al. Cold Spring Harb Symp Quant Biol. 1983;48 Pt 2:453-63. doi: 10.1101/sqb.1983.048.01.050. Cold Spring Harb Symp Quant Biol. 1983. PMID: 6586368 - Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons.
Chalfie M, Au M. Chalfie M, et al. Science. 1989 Feb 24;243(4894 Pt 1):1027-33. doi: 10.1126/science.2646709. Science. 1989. PMID: 2646709 Review. - The genetic control of cell lineage during nematode development.
Sternberg PW, Horvitz HR. Sternberg PW, et al. Annu Rev Genet. 1984;18:489-524. doi: 10.1146/annurev.ge.18.120184.002421. Annu Rev Genet. 1984. PMID: 6397125 Review. No abstract available.
Cited by
- Distinct Neural Circuits Control Rhythm Inhibition and Spitting by the Myogenic Pharynx of C. elegans.
Bhatla N, Droste R, Sando SR, Huang A, Horvitz HR. Bhatla N, et al. Curr Biol. 2015 Aug 17;25(16):2075-89. doi: 10.1016/j.cub.2015.06.052. Epub 2015 Jul 23. Curr Biol. 2015. PMID: 26212880 Free PMC article. - Proton Microbeam Targeted Irradiation of the Gonad Primordium Region Induces Developmental Alterations Associated with Heat Shock Responses and Cuticle Defense in Caenorhabditis elegans.
Beaudier P, Devès G, Plawinski L, Dupuy D, Barberet P, Seznec H. Beaudier P, et al. Biology (Basel). 2023 Oct 27;12(11):1372. doi: 10.3390/biology12111372. Biology (Basel). 2023. PMID: 37997971 Free PMC article. - Genes that implement the hermaphrodite mode of dosage compensation in Caenorhabditis elegans.
Plenefisch JD, DeLong L, Meyer BJ. Plenefisch JD, et al. Genetics. 1989 Jan;121(1):57-76. doi: 10.1093/genetics/121.1.57. Genetics. 1989. PMID: 2917714 Free PMC article. - Identification of chemical synapses in the pharynx of Caenorhabditis elegans.
Li H, Avery L, Denk W, Hess GP. Li H, et al. Proc Natl Acad Sci U S A. 1997 May 27;94(11):5912-6. doi: 10.1073/pnas.94.11.5912. Proc Natl Acad Sci U S A. 1997. PMID: 9159174 Free PMC article. - Rapid and permanent neuronal inactivation in vivo via subcellular generation of reactive oxygen with the use of KillerRed.
Williams DC, Bejjani RE, Ramirez PM, Coakley S, Kim SA, Lee H, Wen Q, Samuel A, Lu H, Hilliard MA, Hammarlund M. Williams DC, et al. Cell Rep. 2013 Oct 31;5(2):553-63. doi: 10.1016/j.celrep.2013.09.023. Cell Rep. 2013. PMID: 24209746 Free PMC article.
References
- Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Phil. Trans. Roy. Sot. London Ser. B. 1976;275:299–325. - PubMed
- Chalfie M, Horvitz HR, Sulston JE. Mutations that lead to reiterations in the cell lineages of C. elegans. Cell. 1981;24:59–69. - PubMed
- Croll NA. The Behavior of Nematodes. London: Edward Arnold; 1970.
- Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986;44:817–829. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources