Novel Echinacea formulations for the treatment of acute respiratory tract infections in adults-A randomized blinded controlled trial - PubMed (original) (raw)
doi: 10.3389/fmed.2023.948787. eCollection 2023.
Karin Keckeis # 1, Giulia Scanferla 1, Manuel Frischknecht 1, Julia Notter 1, Ana Steffen 1, Philipp Kohler 1, Patrick Schmid 1, Bianca Roth 2, Kerstin Wissel 3, Pietro Vernazza 1, Peter Klein 4, Roland Schoop 5, Werner C Albrich 1
Affiliations
- PMID: 37138742
- PMCID: PMC10150997
- DOI: 10.3389/fmed.2023.948787
Novel Echinacea formulations for the treatment of acute respiratory tract infections in adults-A randomized blinded controlled trial
Johannes Sumer et al. Front Med (Lausanne). 2023.
Abstract
Background: Echinacea purpurea has clinical antiviral activity against respiratory viruses and modulates immune functions. In this study, we compared higher doses of new Echinacea formulations with conventional formulations at lower, preventive doses for therapy of respiratory tract infections (RTIs).
Methods: In this randomized, blinded, controlled trial, healthy adults (n = 409) were randomized between November 2018 and January 2019 to one of four Echinacea formulations, which were taken in case of an RTI for up to 10 days. New formulations A (lozenges) and B (spray) delivered an increased dose of 16,800 mg/d Echinacea extract during days 1-3 and 2,240-3,360 mg/d afterward; as controls, conventional formulations C (tablets) and D (drops) delivered a lower daily dose of 2,400 mg, usually taken for prevention. The primary endpoint was time to clinical remission of first RTI episodes based on the Kaplan-Meier analysis of patient-reported, investigator-confirmed, respiratory symptoms assessed for up to 10 days. In a sensitivity analysis, the mean time to remission beyond day 10 was calculated by extrapolating the treatment effects observed on days 7 to 10.
Results: A total of 246 participants (median age 32 years, 78% female participants) were treated for at least one RTI. Recovery by day 10 (complete absence of symptoms) was achieved in 56 and 44% of patients with the new and conventional formulations, respectively, showing a median time to recovery of 10 and 11 days, respectively (p = 0.10 in intention-to-treat analysis, p = 0.07 in per-protocol analysis). In the extrapolated sensitivity analysis, new formulations resulted in a significantly shorter mean time to remission (9.6 vs. 11.0 days, p < 0.001). Among those with an identified respiratory virus, viral clearance until day 10 based on real-time PCR from nasopharyngeal swabs was more frequent with new formulations (70 vs. 53%, p = 0.046). Tolerability and safety (adverse events: 12 vs. 6%, p = 0.19) were good and similar between formulations. There was one severe adverse event with a potential hypersensitivity reaction in a recipient of the novel spray formulation.
Conclusion: In adults with acute RTI, new Echinacea formulations with higher doses resulted in faster viral clearance than conventional formulations in prophylactic dosages. The trend for faster clinical recovery was not significant by day 10 but became so upon extrapolation. A dose increase during acute respiratory symptoms might improve the clinical benefits of orally administered Echinacea formulations.
Trial registration: The study was registered in the Swiss National Clinical Trials Portal (SNCTP000003069) and on ClinicalTrials.gov (NTC03812900; URL https://clinicaltrials.gov/ct2/show/NCT03812900?cond=echinacea&draw=3&rank=14).
Keywords: Echinacea purpurea; antiviral; pharmaceutical formulations; randomized controlled trial; respiratory tract infection.
Copyright © 2023 Sumer, Keckeis, Scanferla, Frischknecht, Notter, Steffen, Kohler, Schmid, Roth, Wissel, Vernazza, Klein, Schoop and Albrich.
Conflict of interest statement
WA has received a speaker's fee from A. Vogel AG. RS is employed by A. Vogel AG, Switzerland, but was not involved in data collection. Data evaluation was carried out by PKl, an independent provider of data management and statistical services. PKl was an employee of d.s.h. Statistical Services GmbH, Germany. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from A. Vogel AG Switzerland. The funder had the following involvement in the study: preparation of formulation and compounds with support from the Kommission für Technologie und Innovation KTI, project 19002.2PFLS-LS, and statistical analyses.
Figures
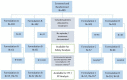
Figure 1
Study flow diagram. Number of patients are shown in both, PP and ITT populations; formulation A, lozenges; formulation B, spray; formulation C, conventional tablets; formulation D, conventional drops; RTI, respiratory tract infection; SAE, severe adverse event. 1Two patients were excluded from PP analysis due to seriously delayed follow-up phone call and medication of obstructive airways disease (exclusion criteria).
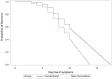
Figure 2
(A) Remission of first infection episode. Comparison of new (dashed line, A + B) vs. conventional (solid line, C + D) formulations in ITT population; x-axis: day free of symptoms; y-axis: the probability of recovery (Kaplan–Meier analysis). (B) Remission of first infection episode extrapolated until full recovery. Comparison of new (dashed line, A + B) versus conventional (solid line, C + D) formulations in ITT population; x-axis: day free of symptoms, y-axis: the probability of recovery (Kaplan–Meier analysis).
Figure 3
Remission of all infection episodes extrapolated until full recovery. Comparison of recovery times of new (dashed line, A + B) vs. conventional (solid line, C + D) (p < 0.001) formulations extrapolated beyond day 10 to absent symptoms; y-axis: the probability of recovery; x-axis: day free of symptoms; the Kaplan–Meier analysis.
Similar articles
- Echinacea Reduces Antibiotics by Preventing Respiratory Infections: A Meta-Analysis (ERA-PRIMA).
Gancitano G, Mucci N, Stange R, Ogal M, Vimalanathan S, Sreya M, Booker A, Hadj-Cherif B, Albrich WC, Woelkart-Ardjomand K, Kreft S, Vanden Berghe W, Hoexter G, Schapowal A, Johnston SL. Gancitano G, et al. Antibiotics (Basel). 2024 Apr 16;13(4):364. doi: 10.3390/antibiotics13040364. Antibiotics (Basel). 2024. PMID: 38667040 Free PMC article. Review. - Echinacea reduces antibiotic usage in children through respiratory tract infection prevention: a randomized, blinded, controlled clinical trial.
Ogal M, Johnston SL, Klein P, Schoop R. Ogal M, et al. Eur J Med Res. 2021 Apr 8;26(1):33. doi: 10.1186/s40001-021-00499-6. Eur J Med Res. 2021. PMID: 33832544 Free PMC article. Clinical Trial. - Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.
Emadi A, Chua JV, Talwani R, Bentzen SM, Baddley J. Emadi A, et al. Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article. - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - Echinacea as a Potential Force against Coronavirus Infections? A Mini-Review of Randomized Controlled Trials in Adults and Children.
Nicolussi S, Ardjomand-Woelkart K, Stange R, Gancitano G, Klein P, Ogal M. Nicolussi S, et al. Microorganisms. 2022 Jan 19;10(2):211. doi: 10.3390/microorganisms10020211. Microorganisms. 2022. PMID: 35208665 Free PMC article. Review.
Cited by
- Echinacea Reduces Antibiotics by Preventing Respiratory Infections: A Meta-Analysis (ERA-PRIMA).
Gancitano G, Mucci N, Stange R, Ogal M, Vimalanathan S, Sreya M, Booker A, Hadj-Cherif B, Albrich WC, Woelkart-Ardjomand K, Kreft S, Vanden Berghe W, Hoexter G, Schapowal A, Johnston SL. Gancitano G, et al. Antibiotics (Basel). 2024 Apr 16;13(4):364. doi: 10.3390/antibiotics13040364. Antibiotics (Basel). 2024. PMID: 38667040 Free PMC article. Review.
References
- Antibiotic Use for Viral Acute Respiratory Tract Infections Remains Common. AJMC. Available online at: https://www.ajmc.com/view/antibiotic-use-for-viral-acute-respiratory-tra... (accessed September 18, 2020). - PubMed
Grants and funding
The authors declare that this study received funding from the Swiss Innovation Agency (project nr. 19002.2) and A. Vogel AG Switzerland.
LinkOut - more resources
Full Text Sources