The interrelationship between sleep, diet, and glucose metabolism - PubMed (original) (raw)
Review
The interrelationship between sleep, diet, and glucose metabolism
Marie-Pierre St-Onge et al. Sleep Med Rev. 2023 Jun.
Abstract
Obesity and type 2 diabetes (T2D) are increasingly common worldwide. While these disorders have increased in prevalence over the past several decades, there has been a concomitant reduction in sleep duration. Short sleep duration has been associated with higher rates of obesity and T2D, and the causality of these associations and their directionality, continue to necessitate evaluation. In this review we consider the evidence that sleep is an intrinsic factor in the development of obesity and chronic metabolic disorders, such as insulin resistance and T2D, while evaluating a potential bi-directional association. We consider the evidence that diet and meal composition, which are known to impact glycemic control, may have both chronic and acute impact upon sleep. Moreover, we consider that postprandial nocturnal metabolism and peripheral glycemia may affect sleep quality. We propose putative mechanisms whereby acute effects of nighttime glucose excursions may lead to increased sleep fragmentation. We conclude that dietary manipulations, particularly with respect to carbohydrate quality, may confer sleep benefits. Future research may seek to evaluate the effectiveness of synergistic nutrient strategies to promote sleep quality, with particular attention to carbohydrate quality, quantity, and availability as well as carbohydrate to protein ratio.
Keywords: Diet; Energy balance; Food intake; Glucose; Obesity; Orexin; Type 2 diabetes.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Figures
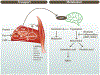
Figure 1.
Simplified illustration of the ‘Energy balance model’ and the ‘Carbohydrate – insulin model’ of obesity. 1a. The energy balance model proposes that changes in the food environment as well as internal signals (hormones) driven by food intake, are cues to the brain which lead to increased food intake. These signals are integrated in the brain modulating behavour reinforcing of food reward, appetite which leads to more food intake and circulating fuels. Circulating fuels and signals (e.g. leptin) indicate the energy status of various organs and are sensed by the brain to control food intake. Overall this model proposes that a genetic variation in the handling of these different processes, particularly those in the brain, are responsible for the susceptibility to develop obesity. 1b. The carbohydrate- insulin model proposes that rapid absorption of glucose after consumption of a high-GL meal increases secretion of insulin to glucagon ratio, and elicits a glucose-dependent insulinotropic polypeptide (GIP)-dominant incretin response. These inputs and/or higher insulin sensitivity in adipose tissue vs. liver or muscle lead to increased lipogenesis and fat storage. The sharp insulin response and decline in blood glucose, possibly below baseline is interpreted in the brains as a state of “cellular semistarvation” and responds with a counter-regulatory hormone response and hunger and cravings for high-GL foods. Energy expenditure may also decline related to decreased fuel availability. Overall, this model proposes that fat storage due to high insulin sensitivity and cellular semistarvation lead to obesity. GI; gastrointestinal; GIP; glucose-dependent insulinotropic polypeptide; GL, glycemic load.
Figure 2.
Transport and metabolism of dietary Trp in the CNS. Trp is derived from dietary protein along with other LNAAs. Transport of Trp/ LNAAs compete at Large Neutral Amino Acid Transporter (LAT1) sites. CHO ingestion leads to insulin secretion and dietary fat increases CCK which may also induce insulin secretion. Elevated insulin drives LNAA into muscle tissue excluding Trp. Trp transport into the brain is thus upregulated. In the brain Trp is metabolized to serotonin and melatonin or is metabolized via the kynurenine pathway. In individuals with obesity-induced inflammation leading to IR, the kynurenine pathway is upregulated. Diagram adapted from Cheon & Kim (67) CCK, cholecystokinin; CHO, carbohydrate; CNS, central nervous system; IDO, indolamine-2,3-dioxygenase; IR, insulin resistance; Trp; Tryptophan; LAT1, large neutral amino acid transporter 1; LNAA, large neutral amino acids.
Similar articles
- Diabetes and branched-chain amino acids: What is the link?
Bloomgarden Z. Bloomgarden Z. J Diabetes. 2018 May;10(5):350-352. doi: 10.1111/1753-0407.12645. Epub 2018 Feb 13. J Diabetes. 2018. PMID: 29369529 - Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability.
Chang CR, Francois ME, Little JP. Chang CR, et al. Am J Clin Nutr. 2019 May 1;109(5):1302-1309. doi: 10.1093/ajcn/nqy261. Am J Clin Nutr. 2019. PMID: 30968140 Free PMC article. Clinical Trial. - Role of High Energy Breakfast "Big Breakfast Diet" in Clock Gene Regulation of Postprandial Hyperglycemia and Weight Loss in Type 2 Diabetes.
Jakubowicz D, Wainstein J, Tsameret S, Landau Z. Jakubowicz D, et al. Nutrients. 2021 May 5;13(5):1558. doi: 10.3390/nu13051558. Nutrients. 2021. PMID: 34063109 Free PMC article. Review. - Acute Effects of Dietary Carbohydrate Restriction on Glycemia, Lipemia and Appetite Regulating Hormones in Normal-Weight to Obese Subjects.
Samkani A, Skytte MJ, Thomsen MN, Astrup A, Deacon CF, Holst JJ, Madsbad S, Rehfeld JF, Krarup T, Haugaard SB. Samkani A, et al. Nutrients. 2018 Sep 12;10(9):1285. doi: 10.3390/nu10091285. Nutrients. 2018. PMID: 30213037 Free PMC article. Clinical Trial. - Sleep duration and disorders in pregnancy: implications for glucose metabolism and pregnancy outcomes.
O'Keeffe M, St-Onge MP. O'Keeffe M, et al. Int J Obes (Lond). 2013 Jun;37(6):765-70. doi: 10.1038/ijo.2012.142. Epub 2012 Sep 4. Int J Obes (Lond). 2013. PMID: 22945608 Free PMC article. Review.
Cited by
- Habitual Short Sleep Duration, Diet, and Development of Type 2 Diabetes in Adults.
Nôga DA, Meth EMES, Pacheco AP, Tan X, Cedernaes J, van Egmond LT, Xue P, Benedict C. Nôga DA, et al. JAMA Netw Open. 2024 Mar 4;7(3):e241147. doi: 10.1001/jamanetworkopen.2024.1147. JAMA Netw Open. 2024. PMID: 38441893 Free PMC article. - Development and Validation of a Nocturnal Hypoglycaemia Risk Model for Patients With Type 2 Diabetes Mellitus.
Gong C, Cai T, Wang Y, Xiong X, Zhou Y, Zhou T, Sun Q, Huang H. Gong C, et al. Nurs Open. 2024 Oct;11(10):e70055. doi: 10.1002/nop2.70055. Nurs Open. 2024. PMID: 39363560 Free PMC article. - Association of Physical Activity and/or Diet with Sleep Quality and Duration in Adolescents: A Scoping Review.
Cruz J, Llodio I, Iturricastillo A, Yanci J, Sánchez-Díaz S, Romaratezabala E. Cruz J, et al. Nutrients. 2024 Oct 1;16(19):3345. doi: 10.3390/nu16193345. Nutrients. 2024. PMID: 39408312 Free PMC article. Review. - A sweet spot for the sleeping brain: Linking human sleep physiology and glucoregulation.
Niethard N, Hallschmid M. Niethard N, et al. Cell Rep Med. 2023 Jul 18;4(7):101123. doi: 10.1016/j.xcrm.2023.101123. Cell Rep Med. 2023. PMID: 37467713 Free PMC article. - Daily variation in blood glucose levels during continuous enteral nutrition in patients on the intensive care unit: a retrospective observational study.
Hiemstra FW, Stenvers DJ, Kalsbeek A, de Jonge E, van Westerloo DJ, Kervezee L. Hiemstra FW, et al. EBioMedicine. 2024 Jun;104:105169. doi: 10.1016/j.ebiom.2024.105169. Epub 2024 May 30. EBioMedicine. 2024. PMID: 38821022 Free PMC article.
References
- Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018. NCHS Health E-Stats 2020.
- Hall KD, Farooqi IS, Friedman JM, Klein S, Loos RJF, Mangelsdorf DJ, O’Rahilly S, Ravussin E, Redman LM, Ryan DH, et al. The energy balance model of obesity: beyond calories in, calories out. The American journal of clinical nutrition 2022;115(5):1243–54. doi: 10.1093/ajcn/nqac031. - DOI - PMC - PubMed
- Ludwig DS, Aronne LJ, Astrup A, de Cabo R, Cantley LC, Friedman MI, Heymsfield SB, Johnson JD, King JC, Krauss RM, et al. The carbohydrate-insulin model: a physiological perspective on the obesity pandemic. The American journal of clinical nutrition 2021;114(6):1873–85. doi: 10.1093/ajcn/nqab270. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous