The emerging spectrum of fetal acetylcholine receptor antibody-related disorders (FARAD) - PubMed (original) (raw)
. 2023 Oct 3;146(10):4233-4246.
doi: 10.1093/brain/awad153.
Mark O'Rahelly 1, Bruno Eymard 2, Mondher Chouchane 3, Andreas Hahn 4, Gerry Kearns 5, Dae-Seong Kim 6, Shin Yun Byun 7, Cam-Tu Emilie Nguyen 8, Ulrike Schara-Schmidt 9, Heike Kölbel 9, Adela Della Marina 9, Christiane Schneider-Gold 10, Kathryn Roefke 11, Andrea Thieme 12, Peter Van den Bergh 13, Gloria Avalos 14, Rodrigo Álvarez-Velasco 15, Daniel Natera-de Benito 16, Man Hin Mark Cheng 17, Wing Ki Chan 17, Hoi Shan Wan 17, Mary Ann Thomas 18, Lauren Borch 18, Julie Lauzon 18, Cornelia Kornblum 19 20, Jens Reimann 19, Andreas Mueller 21, Thierry Kuntzer 22, Fiona Norwood 23, Sithara Ramdas 24, Leslie W Jacobson 25, Xiaobo Jie 25, Miguel A Fernandez-Garcia 26, Elizabeth Wraige 26, Ming Lim 26 27, Jean Pierre Lin 26, Kristl G Claeys 28 29, Selma Aktas 30, Maryam Oskoui 31 32 33, Yael Hacohen 34 35, Ameneh Masud 36 37, M Isabel Leite 25, Jacqueline Palace 25, Darryl De Vivo 36 37, Angela Vincent 25, Heinz Jungbluth 26 38
Affiliations
- PMID: 37186601
- PMCID: PMC10545502
- DOI: 10.1093/brain/awad153
The emerging spectrum of fetal acetylcholine receptor antibody-related disorders (FARAD)
Nicholas M Allen et al. Brain. 2023.
Abstract
In utero exposure to maternal antibodies targeting the fetal acetylcholine receptor isoform (fAChR) can impair fetal movement, leading to arthrogryposis multiplex congenita (AMC). Fetal AChR antibodies have also been implicated in apparently rare, milder myopathic presentations termed fetal acetylcholine receptor inactivation syndrome (FARIS). The full spectrum associated with fAChR antibodies is still poorly understood. Moreover, since some mothers have no myasthenic symptoms, the condition is likely underreported, resulting in failure to implement effective preventive strategies. Here we report clinical and immunological data from a multicentre cohort (n = 46 cases) associated with maternal fAChR antibodies, including 29 novel and 17 previously reported with novel follow-up data. Remarkably, in 50% of mothers there was no previously established myasthenia gravis (MG) diagnosis. All mothers (n = 30) had AChR antibodies and, when tested, binding to fAChR was often much greater than that to the adult AChR isoform. Offspring death occurred in 11/46 (23.9%) cases, mainly antenatally due to termination of pregnancy prompted by severe AMC (7/46, 15.2%), or during early infancy, mainly from respiratory failure (4/46, 8.7%). Weakness, contractures, bulbar and respiratory involvement were prominent early in life, but improved gradually over time. Facial (25/34; 73.5%) and variable peripheral weakness (14/32; 43.8%), velopharyngeal insufficiency (18/24; 75%) and feeding difficulties (16/36; 44.4%) were the most common sequelae in long-term survivors. Other unexpected features included hearing loss (12/32; 37.5%), diaphragmatic paresis (5/35; 14.3%), CNS involvement (7/40; 17.5%) and pyloric stenosis (3/37; 8.1%). Oral salbutamol used empirically in 16/37 (43.2%) offspring resulted in symptom improvement in 13/16 (81.3%). Combining our series with all previously published cases, we identified 21/85 mothers treated with variable combinations of immunotherapies (corticosteroids/intravenous immunoglobulin/plasmapheresis) during pregnancy either for maternal MG symptom control (12/21 cases) or for fetal protection (9/21 cases). Compared to untreated pregnancies (64/85), maternal treatment resulted in a significant reduction in offspring deaths (P < 0.05) and other complications, with treatment approaches involving intravenous immunoglobulin/ plasmapheresis administered early in pregnancy most effective. We conclude that presentations due to in utero exposure to maternal (fetal) AChR antibodies are more common than currently recognized and may mimic a wide range of neuromuscular disorders. Considering the wide clinical spectrum and likely diversity of underlying mechanisms, we propose 'fetal acetylcholine receptor antibody-related disorders' (FARAD) as the most accurate term for these presentations. FARAD is vitally important to recognize, to institute appropriate management strategies for affected offspring and to improve outcomes in future pregnancies. Oral salbutamol is a symptomatic treatment option in survivors.
Keywords: arthrogryposis multiplex congenita; congenital myopathy; fetal acetylcholine receptor inactivation syndrome; myasthenia gravis; salbutamol; transient neonatal myasthenia gravis.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures
Figure 1
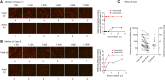
Clinical features in offspring with fetal acetylcholine receptor antibody-related disorders (FARAD) at different time points. The offspring of myasthenic mothers with antibodies to the (fetal) acetylcholine receptor has a wide spectrum of features, ranging from lethal arthrogryposis multiplex congenita (AMC) (A–D) to less severe contracture phenotypes (F and H–J). Congenital hypotonia and facial weakness (E) are common. Respiratory impairment requiring (non-invasive) ventilation (G) and bulbar involvement requiring nasogastric tube feeds (F, G and L–N) are common. Less common features include diaphragmatic paresis (K) and extraocular muscle involvement (L). Later in life, persistent facial weakness with a myopathic facial appearance (O, P and T–V, and X), incomplete eye closure and an inverted V-shaped mouth are common. Facial weakness may at least be partially responsive to treatment with oral salbutamol (W). Significant jaw contractures (Q and R, 3D CT reconstruction) in more severely affected patients, and scoliosis (Y and Z, 3D CT reconstruction) can occur. A small proportion of children may have a longer ventilatory and enteral feeding requirement (N).
Figure 2
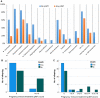
Maternal acetylcholine receptor antibody results. Fetal (f) and adult (ad) acetylcholine receptor (AChR) antibody titres varied considerably. The figure shows representative cell-based assays (CBAs) from two mothers with (A, Case 2.1) very high fAChR binding scoring 3 even at 1:1600 dilution compared with almost undetectable binding to adAChR at 1:50 dilution; the mother shown in B (Case 9) had less fAChR antibodies but more adAChR antibodies than the mother in A. Titres of all sera binding to fetal (red) and adult (black) AChRs, tested by CBAs, are shown in C, with the ratio of fetal to adult titres illustrating the variability.
Figure 3
Correlation between maternal immunotherapy during pregnancy and outcome in offspring. (A) Comparison of overall rate (%) and reduction of major clinical features (complications) in the maternally-administered immunotherapy during pregnancy (pIMT) group compared to those with no pIMT (*P < 0.05). (B) Devised score to reflect the mode and timing of treatment during pregnancy (Supplementary Table 3) showing the relative proportion of deaths decreased with the intensity of treatment (P < 0.05), and (C) no deaths observed with a treatment score above 15. Similar trends were seen for other major features (Table 3 and Supplementary Tables 2 and 5).
Figure 4
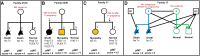
Illustrative case scenarios showing effect of pregnancy immunotherapy on outcome. The main determinants of outcome are the mode and timing of immunotherapy during pregnancy, with plasmapheresis (PLEX) and intravenous immunoglobulin (IVIG), alone or in combination with moderate-high dose steroids, early in the pregnancy most effective. Poor outcomes/death seen with less effective (or ineffective) interventions; intermediate outcomes are highlighted in orange; good outcomes are clear. (A) Family 41(H) illustrates poor outcome [severe arthrogryposis multiplex congenita (AMC) and death] in two untreated pregnancies, but good outcome in the third pregnancy treated with combination of high dose prednisolone and PLEX in early gestation (aTNMG-like but details of longer follow-up not available). (B) Family 23(F) illustrates better outcome in the second (orange) and good outcome in the third pregnancies treated with IVIG and PLEX from Trimester 1 onward respectively, compared to the first pregnancy with poor outcome, where similar treatments were administered only from Trimester 3 onwards. (C) Family 11 illustrates that the outcome may not be invariably severe even in untreated pregnancies (Case 11.1 with a myopathic non-AMC phenotype following untreated pregnancy) but may still improve further in appropriately treated subsequent pregnancies (Case 11.2, normal at 10 years follow-up). (D) Family 17 illustrates a (same-sex) couple who had two previous lethal AMC-affected untreated pregnancies, using ovum from the unaffected (green) partner, to the undiagnosed fAChR antibody-carrying partner/pregnancy (blue), but subsequently used the ovum from the fAChR antibody-carrying partner (blue) for surrogacy to the antibody negative, unaffected partner (green) achieving uncomplicated healthy third and fourth pregnancies (Offspring S1 and S2). D1/D2 = sperm donors; dx = diagnosis; F = follow-up cases, new data; H = historical cases, no new follow-up data; MG = myasthenia gravis; pIMT = pregnancy immunotherapy treatment; Pred. = prednisolone; S = surrogacy; T = trimester; Tx = treatment. See Supplementary Table 2A for all treated cases and dose regimes and Supplementary Table 2B (Table view of Fig. 4).
Similar articles
- Maternal immunoglobulin treatment can reduce severity of fetal acetylcholine receptor antibody-associated disorders (FARAD).
Wassenberg M, Hahn A, Mück A, Krämer HH. Wassenberg M, et al. Neurol Res Pract. 2023 Oct 26;5(1):58. doi: 10.1186/s42466-023-00280-6. Neurol Res Pract. 2023. PMID: 37880783 Free PMC article. - Teratogen update: maternal myasthenia gravis as a cause of congenital arthrogryposis.
Polizzi A, Huson SM, Vincent A. Polizzi A, et al. Teratology. 2000 Nov;62(5):332-41. doi: 10.1002/1096-9926(200011)62:5<332::AID-TERA7>3.0.CO;2-E. Teratology. 2000. PMID: 11029151 Review. - Association of arthrogryposis multiplex congenita with maternal antibodies inhibiting fetal acetylcholine receptor function.
Riemersma S, Vincent A, Beeson D, Newland C, Hawke S, Vernet-der Garabedian B, Eymard B, Newsom-Davis J. Riemersma S, et al. J Clin Invest. 1996 Nov 15;98(10):2358-63. doi: 10.1172/JCI119048. J Clin Invest. 1996. PMID: 8941654 Free PMC article. - Fetal acetylcholine receptor inactivation syndrome: A myopathy due to maternal antibodies.
Hacohen Y, Jacobson LW, Byrne S, Norwood F, Lall A, Robb S, Dilena R, Fumagalli M, Born AP, Clarke D, Lim M, Vincent A, Jungbluth H. Hacohen Y, et al. Neurol Neuroimmunol Neuroinflamm. 2014 Dec 23;2(1):e57. doi: 10.1212/NXI.0000000000000057. eCollection 2015 Feb. Neurol Neuroimmunol Neuroinflamm. 2014. PMID: 25566546 Free PMC article. - Antibodies affecting ion channel function in acquired neuromyotonia, in seropositive and seronegative myasthenia gravis, and in antibody-mediated arthrogryposis multiplex congenita.
Vincent A, Jacobson L, Plested P, Polizzi A, Tang T, Riemersma S, Newland C, Ghorazian S, Farrar J, MacLennan C, Willcox N, Beeson D, Newsom-Davis J. Vincent A, et al. Ann N Y Acad Sci. 1998 May 13;841:482-96. doi: 10.1111/j.1749-6632.1998.tb10968.x. Ann N Y Acad Sci. 1998. PMID: 9668280 Review.
Cited by
- Advancing fetal diagnosis and prognostication using comprehensive prenatal phenotyping and genetic testing.
Fortin O, Mulkey SB, Fraser JL. Fortin O, et al. Pediatr Res. 2024 Jun 27. doi: 10.1038/s41390-024-03343-9. Online ahead of print. Pediatr Res. 2024. PMID: 38937640 Review. - Maternal immunoglobulin treatment can reduce severity of fetal acetylcholine receptor antibody-associated disorders (FARAD).
Wassenberg M, Hahn A, Mück A, Krämer HH. Wassenberg M, et al. Neurol Res Pract. 2023 Oct 26;5(1):58. doi: 10.1186/s42466-023-00280-6. Neurol Res Pract. 2023. PMID: 37880783 Free PMC article. - Pregnancy in myasthenia gravis: a retrospective analysis of maternal and neonatal outcome from a large tertiary care centre in Germany.
Draxler J, Meisel A, Stascheit F, Stein M, Gerischer L, Mergenthaler P, Herdick M, Doksani P, Lehnerer S, Verlohren S, Hoffmann S. Draxler J, et al. Arch Gynecol Obstet. 2024 Jul;310(1):277-284. doi: 10.1007/s00404-024-07436-y. Epub 2024 Mar 16. Arch Gynecol Obstet. 2024. PMID: 38492082 Free PMC article. - Case report: A highly active refractory myasthenia gravis with treatment of telitacicept combined with efgartigimod.
Zhang C, Lin Y, Kuang Q, Li H, Jiang Q, Yang X. Zhang C, et al. Front Immunol. 2024 May 10;15:1400459. doi: 10.3389/fimmu.2024.1400459. eCollection 2024. Front Immunol. 2024. PMID: 38799457 Free PMC article. - Transient Neonatal Myasthenia Gravis as a Common Complication of a Rare Disease: A Systematic Review.
Lindroos JLV, Bjørk MH, Gilhus NE. Lindroos JLV, et al. J Clin Med. 2024 Feb 17;13(4):1136. doi: 10.3390/jcm13041136. J Clin Med. 2024. PMID: 38398450 Free PMC article. Review.
References
- Hesselmans LF, Jennekens FG, Van den Oord CJ, Veldman H, Vincent A. Development of innervation of skeletal muscle fibers in man: Relation to acetylcholine receptors. Anat Rec. 1993;236:553–562. - PubMed
- Barnes PR, Kanabar DJ, Brueton L, et al. . Recurrent congenital arthrogryposis leading to a diagnosis of myasthenia gravis in an initially asymptomatic mother. Neuromuscul Disord. 1995;5:59–65. - PubMed
- Brueton LA, Huson SM, Cox PM, et al. . Asymptomatic maternal myasthenia as a cause of the Pena-Shokeir phenotype. Am J Med Genet. 2000;92:1–6. - PubMed
- Shepard MK. Arthrogryposis multiplex congenita in sibs. Birth Defects Orig Artic Ser. 1971;7:127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical