Frailty in randomised controlled trials for dementia or mild cognitive impairment measured via the frailty index: prevalence and prediction of serious adverse events and attrition - PubMed (original) (raw)
Meta-Analysis
Frailty in randomised controlled trials for dementia or mild cognitive impairment measured via the frailty index: prevalence and prediction of serious adverse events and attrition
Heather Wightman et al. Alzheimers Res Ther. 2023.
Abstract
Background: Frailty and dementia have a bidirectional relationship. However, frailty is rarely reported in clinical trials for dementia and mild cognitive impairment (MCI) which limits assessment of trial applicability. This study aimed to use a frailty index (FI)-a cumulative deficit model of frailty-to measure frailty using individual participant data (IPD) from clinical trials for MCI and dementia. Moreover, the study aimed to quantify the prevalence of frailty and its association with serious adverse events (SAEs) and trial attrition.
Methods: We analysed IPD from dementia (n = 1) and MCI (n = 2) trials. An FI comprising physical deficits was created for each trial using baseline IPD. Poisson and logistic regression were used to examine associations with SAEs and attrition, respectively. Estimates were pooled in random effects meta-analysis. Analyses were repeated using an FI incorporating cognitive as well as physical deficits, and results compared.
Results: Frailty could be estimated in all trial participants. The mean physical FI was 0.14 (SD 0.06) and 0.14 (SD 0.06) in the MCI trials and 0.24 (SD 0.08) in the dementia trial. Frailty prevalence (FI > 0.24) was 6.9%/7.6% in MCI trials and 48.6% in the dementia trial. After including cognitive deficits, the prevalence was similar in MCI (6.1% and 6.7%) but higher in dementia (75.4%). The 99th percentile of FI (0.31 and 0.30 in MCI, 0.44 in dementia) was lower than in most general population studies. Frailty was associated with SAEs: physical FI IRR = 1.60 [1.40, 1.82]; physical/cognitive FI IRR = 1.64 [1.42, 1.88]. In a meta-analysis of all three trials, the estimated association between frailty and trial attrition included the null (physical FI OR = 1.17 [0.92, 1.48]; physical/cognitive FI OR = 1.16 [0.92, 1.46]), although higher frailty index values were associated with attrition in the dementia trial.
Conclusion: Measuring frailty from baseline IPD in dementia and MCI trials is feasible. Those living with more severe frailty may be under-represented. Frailty is associated with SAEs. Including only physical deficits may underestimate frailty in dementia. Frailty can and should be measured in future and existing trials for dementia and MCI, and efforts should be made to facilitate inclusion of people living with frailty.
Keywords: Cognitive impairment; Dementia; Frailty; Randomised controlled trials.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
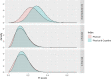
Fig. 1
Distribution of the frailty index (comparing ‘physical’ and ‘physical & cognitive’ frailty indices): This figure shows the distribution of frailty in each of the included trials, as modelled using a generalised gamma distribution. Pink shading indicates the frailty index comprised only of physical deficits, and blue shading indicates the frailty index combining physical and cognitive deficits
Fig. 2
Association between frailty and incidence of serious adverse events. This figure shows the results of a random effects meta-analysis of the association between frailty index and incident serious adverse events. Incidence rate ratios (with 95% confidence intervals) show the results per 0.1-point increase in the frailty index
Fig. 3
Association between frailty and odds of trial attrition. This figure shows the results of a random effects meta-analysis of the association between frailty index and trial attrition. Odds ratios (with 95% confidence intervals) show the results per 0.1-point increase in the frailty index
Similar articles
- Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force [Internet].
Patnode CD, Perdue LA, Rossom RC, Rushkin MC, Redmond N, Thomas RG, Lin JS. Patnode CD, et al. Rockville (MD): Agency for Healthcare Research and Quality (US); 2020 Feb. Report No.: 19-05257-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2020 Feb. Report No.: 19-05257-EF-1. PMID: 32129963 Free Books & Documents. Review. - Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force [Internet].
Lin JS, O'Connor E, Rossom RC, Perdue LA, Burda BU, Thompson M, Eckstrom E. Lin JS, et al. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Nov. Report No.: 14-05198-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Nov. Report No.: 14-05198-EF-1. PMID: 24354019 Free Books & Documents. Review. - Identifying frailty in trials: an analysis of individual participant data from trials of novel pharmacological interventions.
Hanlon P, Butterly E, Lewsey J, Siebert S, Mair FS, McAllister DA. Hanlon P, et al. BMC Med. 2020 Oct 22;18(1):309. doi: 10.1186/s12916-020-01752-1. BMC Med. 2020. PMID: 33087107 Free PMC article. - Sex Moderates the Association between Frailty and Mild Behavioral Impairment.
Guan DX, Rockwood K, Smith EE, Ismail Z. Guan DX, et al. J Prev Alzheimers Dis. 2022;9(4):692-700. doi: 10.14283/jpad.2022.61. J Prev Alzheimers Dis. 2022. PMID: 36281673 - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
- Frailty Indices and Their Importance in Elderly Patients: A Perspective Review.
Valvani R, Javed N, Vittorio T, Mohyeldin M. Valvani R, et al. J Community Hosp Intern Med Perspect. 2024 Jul 2;14(4):25-33. doi: 10.55729/2000-9666.1344. eCollection 2024. J Community Hosp Intern Med Perspect. 2024. PMID: 39391119 Free PMC article. Review. - Dissecting the shared genetic architecture between Alzheimer's disease and frailty: a cross-trait meta-analyses of genome-wide association studies.
Enduru N, Fernandes BS, Zhao Z. Enduru N, et al. Front Genet. 2024 Apr 19;15:1376050. doi: 10.3389/fgene.2024.1376050. eCollection 2024. Front Genet. 2024. PMID: 38706793 Free PMC article. - To Be Frail or Not to Be Frail: This Is the Question-A Critical Narrative Review of Frailty.
Sciacchitano S, Carola V, Nicolais G, Sciacchitano S, Napoli C, Mancini R, Rocco M, Coluzzi F. Sciacchitano S, et al. J Clin Med. 2024 Jan 26;13(3):721. doi: 10.3390/jcm13030721. J Clin Med. 2024. PMID: 38337415 Free PMC article. Review. - Emerging disease modifying therapies for older adults with Alzheimer disease: perspectives from the EuGMS special interest group in dementia.
Dyer AH, Dolphin H, Shenkin SD, Welsh T, Soysal P, Roitto HM, Religa D, Kennelly SP; EuGMS Special Interest Group in Dementia. Dyer AH, et al. Eur Geriatr Med. 2023 Oct;14(5):919-923. doi: 10.1007/s41999-023-00846-2. Eur Geriatr Med. 2023. PMID: 37597074 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical