Health Consequences of Thymus Removal in Adults - PubMed (original) (raw)
Health Consequences of Thymus Removal in Adults
Kameron A Kooshesh et al. N Engl J Med. 2023.
Abstract
Background: The function of the thymus in human adults is unclear, and routine removal of the thymus is performed in a variety of surgical procedures. We hypothesized that the adult thymus is needed to sustain immune competence and overall health.
Methods: We evaluated the risk of death, cancer, and autoimmune disease among adult patients who had undergone thymectomy as compared with demographically matched controls who had undergone similar cardiothoracic surgery without thymectomy. T-cell production and plasma cytokine levels were also compared in a subgroup of patients.
Results: After exclusions, 1420 patients who had undergone thymectomy and 6021 controls were included in the study; 1146 of the patients who had undergone thymectomy had a matched control and were included in the primary cohort. At 5 years after surgery, all-cause mortality was higher in the thymectomy group than in the control group (8.1% vs. 2.8%; relative risk, 2.9; 95% confidence interval [CI], 1.7 to 4.8), as was the risk of cancer (7.4% vs. 3.7%; relative risk, 2.0; 95% CI, 1.3 to 3.2). Although the risk of autoimmune disease did not differ substantially between the groups in the overall primary cohort (relative risk, 1.1; 95% CI, 0.8 to 1.4), a difference was found when patients with preoperative infection, cancer, or autoimmune disease were excluded from the analysis (12.3% vs. 7.9%; relative risk, 1.5; 95% CI, 1.02 to 2.2). In an analysis involving all patients with more than 5 years of follow-up (with or without a matched control), all-cause mortality was higher in the thymectomy group than in the general U.S. population (9.0% vs. 5.2%), as was mortality due to cancer (2.3% vs. 1.5%). In the subgroup of patients in whom T-cell production and plasma cytokine levels were measured (22 in the thymectomy group and 19 in the control group; mean follow-up, 14.2 postoperative years), those who had undergone thymectomy had less new production of CD4+ and CD8+ lymphocytes than controls (mean CD4+ signal joint T-cell receptor excision circle [sjTREC] count, 1451 vs. 526 per microgram of DNA [P = 0.009]; mean CD8+ sjTREC count, 1466 vs. 447 per microgram of DNA [P<0.001]) and higher levels of proinflammatory cytokines in the blood.
Conclusions: In this study, all-cause mortality and the risk of cancer were higher among patients who had undergone thymectomy than among controls. Thymectomy also appeared be associated with an increased risk of autoimmune disease when patients with preoperative infection, cancer, or autoimmune disease were excluded from the analysis. (Funded by the Tracey and Craig A. Huff Harvard Stem Cell Institute Research Support Fund and others.).
Copyright © 2023 Massachusetts Medical Society.
Figures
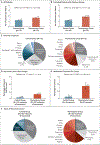
Figure 1.. Effect of Thymectomy on Long-Term Mortality.
Panel A shows the relative risk of death from any cause among patients who had undergone thymectomy as compared with age-, race-, and sex-matched controls in the first 5 years after surgery, both in the overall study population and in subgroups. The relative risk of death from any cause within 5 years was increased in patients who had undergone thymectomy, regardless of whether they had a preoperative history of infection, cancer, or autoimmune disease. Panels B and C show the percentages of patients who survived over a period of 20 years after surgery in the group with no exclusions (Panel B) and in the subgroup in which patients with a preoperative history of infection, cancer, or autoimmune disease were excluded from the analysis. The insets show the same data on an expanded y axis.
Figure 2.. Effect of Thymectomy on the Long-Term Risk of Cancer.
Panel A shows the relative risk of cancer among patients who had undergone thymectomy as compared with age-, race-, and sex-matched controls in the first 5 years after surgery, both in the overall study population and in subgroups. The relative risk of cancer within 5 years was increased in patients who had undergone thymectomy, regardless of whether they had a preoperative history of infection, cancer, or autoimmune disease. Panels B and C show the percentages of patients who survived without cancer over a period of 20 years after surgery in the group with no exclusions (Panel B) and in the subgroup in which patients with a preoperative history of infection, cancer, or autoimmune disease were excluded from the analysis. The insets show the same data on an expanded y axis.
Figure 3.. Characteristics of Post-Thymectomy Cancer (Chart-Review Cohort).
Among the patients with at least one cancer after surgery, the number of cancers per patient was larger, on average, among those who had undergone thymectomy than among controls, regardless of the preoperative history of cancer. Detailed medical record reviews were performed for 75 patients who had undergone thymectomy and 75 controls randomly chosen from the subgroup who had postoperative cancer (chart-review cohort). Panel A shows data for all patients in the cohort, and Panel B shows data for the cohort minus patients with a preoperative history of cancer. Panel C shows the types of cancer that developed after surgery; 64.9% of the cancers in the control group were skin cancers, whereas only 46.5% of the cancers in the thymectomy group were skin cancers. The remainder of the cancers in the thymectomy group represented most major tissue types in the body; those found in more than 1% of the patients in the group are listed next to the pie chart. The total numbers of types of postoperative cancer in the chart-review cohort were 116 in the thymectomy group and 91 in the control group. CLL denotes chronic lymphocytic leukemia, and CNS central nervous system. Panel D shows cancer recurrence after therapy, which was more common in the thymectomy group than in the control group. Panel E shows the percentages of postoperative cancers that would be treated with an intensive regimen involving multimodal therapy (i.e., surgery, chemotherapy, radiation, and monoclonal antibodies) according to National Comprehensive Cancer Network guidelines. Panel F shows the types of cancers that recurred in each group; nonskin cancers that recurred in the thymectomy group are listed next to the pie chart. DLBCL denotes diffuse large B-cell lymphoma.
Figure 4.. Effect of Thymectomy on the Risk of Autoimmune Disease.
The risk of autoimmune disease in the thymectomy group was compared with that among age-, race-, and sex-matched controls in the first 5 years after surgery. The relative risk of autoimmune disease was increased among patients who had undergone thymectomy who did not have a history of preoperative infections, cancers, or autoimmune disease.
Figure 5.. T-Cell Production and Inflammatory Responses.
Panels A and B show an analysis of signal joint T-cell receptor excision circles (sjTRECs) in CD4+ (Panel A) and CD8+ (Panel B) lymphocytes, with results expressed as the sjTREC count per microgram of DNA. CD4+ and CD8+ lymphocytes isolated from patients in the control group had larger mean sjTREC counts than those isolated from patients in the thymectomy group (CD4+: 1451 vs. 526 per microgram of DNA; difference, 925 [95% CI, 82 to 1768]; P = 0.009; and CD8+: 1466 vs. 447 per microgram of DNA; difference, 1019 [95% CI, 330 to 1707]). The CD4+ experiment included 15 patients in the thymectomy group and 17 in the control group; the CD8+ experiment included 11 patients in each group. In the violin plots, dots indicate individual samples and the width of the shaded area indicates the probability density. In each box-and-whisker plot within a violin plot, the horizontal line indicates the median, the top and bottom of the box indicate the interquartile range, and the whiskers indicate 1.5 times the interquartile range. Panel C shows a heat map of cytokines measured in blood plasma obtained from 21 patients who had undergone thymectomy (labeled TMx) and 19 controls. A panel of 71 cytokines was used, 15 of which differed significantly between the groups (P<0.05 with Bonferroni correction). One control (of 19) was excluded from this analysis because anomalous, out-of-range readings were obtained with repeated testing. Bonferroni correction was used for all statistical testing. The figure is based on log10-transformed cytokine concentrations in order to adjust for differences in scales of expression. Unsupervised clustering showed that the patients who had undergone thymectomy had similar cytokine-expression profiles; these patients dominated two clusters, labeled TMx 1 and TMx 2, that differed from the control cluster in their strong expression of type 2 and type 17 helper T-cell cytokines and acute-phase reactants (especially in cluster TMx 1; some patients in cluster TMx 2 shared this phenotype). The z score is highlighted in the top left with a histogram showing the frequency of cytokine expression at different z-score values. EGF denotes epidermal growth factor, FGF fibroblast growth factor, FLT-3L FMS-like tyrosine kinase 3 ligand, G-CSF granulocyte colony-stimulating factor, GM-CSF granulocyte–macrophage colony-stimulating factor, IFN interferon, IL interleukin, LIF leukemia inhibitory factor, M-CSF macrophage colony-stimulating factor, MIG monokine induced by interferon-γ, PDGF platelet-derived growth factor, SCF stem-cell factor, TNF tumor necrosis factor, TPO thrombopoietin, TRAIL tumor necrosis factor–related apoptosis-inducing ligand, TSLP thymic stromal lymphopoietin, and VEGF vascular endothelial growth factor.
Comment in
- Health Consequences of Thymus Removal in Adults.
Kojima T, Uhara K, Mori J. Kojima T, et al. N Engl J Med. 2023 Nov 2;389(18):1724. doi: 10.1056/NEJMc2310640. N Engl J Med. 2023. PMID: 37913513 No abstract available. - Health Consequences of Thymus Removal in Adults.
Morena J; Duke Myasthenia Gravis Physicians. Morena J, et al. N Engl J Med. 2023 Nov 2;389(18):1724-1725. doi: 10.1056/NEJMc2310640. N Engl J Med. 2023. PMID: 37913514 No abstract available. - Health Consequences of Thymus Removal in Adults.
Ilonen I, Kytö V, Kekäläinen E. Ilonen I, et al. N Engl J Med. 2023 Nov 2;389(18):1725-1726. doi: 10.1056/NEJMc2310640. N Engl J Med. 2023. PMID: 37913515 No abstract available. - Health Consequences of Thymus Removal in Adults.
Jeyaranjan R. Jeyaranjan R. N Engl J Med. 2023 Nov 2;389(18):1726. doi: 10.1056/NEJMc2310640. N Engl J Med. 2023. PMID: 37913516 No abstract available. - Health Consequences of Thymus Removal in Adults. Reply.
Kooshesh KA, Gustafsson K, Scadden DT. Kooshesh KA, et al. N Engl J Med. 2023 Nov 2;389(18):1726-1727. doi: 10.1056/NEJMc2310640. N Engl J Med. 2023. PMID: 37913517 No abstract available.
Similar articles
- Mortality impact, risks, and benefits of general population screening for ovarian cancer: the UKCTOCS randomised controlled trial.
Menon U, Gentry-Maharaj A, Burnell M, Ryan A, Kalsi JK, Singh N, Dawnay A, Fallowfield L, McGuire AJ, Campbell S, Skates SJ, Parmar M, Jacobs IJ. Menon U, et al. Health Technol Assess. 2023 May 11:1-81. doi: 10.3310/BHBR5832. Online ahead of print. Health Technol Assess. 2023. PMID: 37183782 Free PMC article. - Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.
Bryant A, Hiu S, Kunonga PT, Gajjar K, Craig D, Vale L, Winter-Roach BA, Elattar A, Naik R. Bryant A, et al. Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review. - Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.
Elwenspoek MM, Thom H, Sheppard AL, Keeney E, O'Donnell R, Jackson J, Roadevin C, Dawson S, Lane D, Stubbs J, Everitt H, Watson JC, Hay AD, Gillett P, Robins G, Jones HE, Mallett S, Whiting PF. Elwenspoek MM, et al. Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - The 2023 Latin America report of the Lancet Countdown on health and climate change: the imperative for health-centred climate-resilient development.
Hartinger SM, Palmeiro-Silva YK, Llerena-Cayo C, Blanco-Villafuerte L, Escobar LE, Diaz A, Sarmiento JH, Lescano AG, Melo O, Rojas-Rueda D, Takahashi B, Callaghan M, Chesini F, Dasgupta S, Posse CG, Gouveia N, Martins de Carvalho A, Miranda-Chacón Z, Mohajeri N, Pantoja C, Robinson EJZ, Salas MF, Santiago R, Sauma E, Santos-Vega M, Scamman D, Sergeeva M, Souza de Camargo T, Sorensen C, Umaña JD, Yglesias-González M, Walawender M, Buss D, Romanello M. Hartinger SM, et al. Lancet Reg Health Am. 2024 Apr 23;33:100746. doi: 10.1016/j.lana.2024.100746. eCollection 2024 May. Lancet Reg Health Am. 2024. PMID: 38800647 Free PMC article. Review.
Cited by
- Active thymus in adult with lung cancer: preliminary results from the Adult Thymic Project.
Sobrero S, Patrucco E, Napoli F, Ragazzini R, Milazzo R, Vaisitti F, Ambrogio C, Bonfanti P, Rena O, Ruffini E, Righi L, Leo F. Sobrero S, et al. Updates Surg. 2024 Aug 21. doi: 10.1007/s13304-024-01953-w. Online ahead of print. Updates Surg. 2024. PMID: 39167357 - Surgical treatment of thymic epithelial tumors: a narrative review.
Agrafiotis AC, Berzenji L, Koyen S, Vermeulen D, Winthagen R, Hendriks JMH, Van Schil PE. Agrafiotis AC, et al. Mediastinum. 2024 Mar 12;8:32. doi: 10.21037/med-23-44. eCollection 2024. Mediastinum. 2024. PMID: 38881810 Free PMC article. Review. - Early-life thymectomy leads to an increase of granzyme-producing γδ T cells in children with congenital heart disease.
Cramer A, Yang T, Riemann L, Almeida V, Kammeyer C, Abu YE, Gluschke E, Kleiner S, León-Lara X, Janssen A, Hofmann A, Horke A, von Kaisenberg C, Förster R, Beerbaum P, Boehne M, Ravens S. Cramer A, et al. Nat Commun. 2024 Nov 13;15(1):9841. doi: 10.1038/s41467-024-51673-3. Nat Commun. 2024. PMID: 39537635 Free PMC article. - Pediatric brain tumor patients display altered immune activation and reduced lymphopoiesis at the onset of disease.
Rosichini M, Del Baldo G, De Luca CD, Benini F, Genah S, Vinci M, Cerimele A, Coccetti M, Flamini S, Carsetti R, Cacchione A, Carai A, Mastronuzzi A, Locatelli F, Velardi E. Rosichini M, et al. NPJ Precis Oncol. 2024 Nov 20;8(1):269. doi: 10.1038/s41698-024-00755-y. NPJ Precis Oncol. 2024. PMID: 39567679 Free PMC article. - Thymus in Cardiometabolic Impairments and Atherosclerosis: Not a Silent Player?
Kologrivova IV, Naryzhnaya NV, Suslova TE. Kologrivova IV, et al. Biomedicines. 2024 Jun 25;12(7):1408. doi: 10.3390/biomedicines12071408. Biomedicines. 2024. PMID: 39061983 Free PMC article. Review.
References
- van Gent R, Schadenberg AWL, Otto SA, et al. Long-term restoration of the human T-cell compartment after thymectomy during infancy: a role for thymic regeneration? Blood 2011;118:627–34. - PubMed
- Wells WJ, Parkman R, Smogorzewska E, Barr M. Neonatal thymectomy: does it affect immune function? J Thorac Cardiovasc Surg 1998;115:1041–6. - PubMed
- Kurobe H, Tominaga T, Sugano M, et al. Complete but not partial thymectomy in early infancy reduces T-cell-mediated immune response: three-year tracing study after pediatric cardiac surgery. J Thorac Cardiovasc Surg 2013;145(3):656–662, 662.e1–662.e2. - PubMed
- Prelog M, Wilk C, Keller M, et al. Diminished response to tick-borne encephalitis vaccination in thymectomized children. Vaccine 2008;26:595–600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials