GABAA receptors as targets for treating affective and cognitive symptoms of depression - PubMed (original) (raw)
Review
GABAA receptors as targets for treating affective and cognitive symptoms of depression
Bernhard Luscher et al. Trends Pharmacol Sci. 2023 Sep.
Abstract
In the past 20 years, our understanding of the pathophysiology of depression has evolved from a focus on an imbalance of monoaminergic neurotransmitters to a multifactorial picture including an improved understanding of the role of glutamatergic excitatory and GABAergic inhibitory neurotransmission. FDA-approved treatments targeting the glutamatergic [esketamine for major depressive disorder (MDD)] and GABAergic (brexanolone for peripartum depression) systems have become available. This review focuses on the GABAA receptor (GABAAR) system as a target for novel antidepressants and discusses the mechanisms by which modulation of δ-containing GABAARs with neuroactive steroids (NASs) or of α5-containing GABAARs results in antidepressant or antidepressant-like actions and discusses clinical data on NASs. Moreover, a potential mechanism by which α5-GABAAR-positive allosteric modulators (PAMs) may improve cognitive deficits in depression is presented.
Keywords: GABA(A) receptor; Gabra5; Gabrd; chronic stress; cognition; depression; emotion; neurosteroids.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests J.L.M. serves on the Scientific Advisory Board and has a sponsored research agreement with SAGE Therapeutics. U.R. is Scientific Advisor to Damona Pharmaceuticals. E.S. is the cofounder and chief scientific officer of DAMONA Pharmaceuticals. He is listed on patents for molecules modulating GABAergic functions, which are licensed to DAMONA Pharmaceuticals. B.L. has no conflict of interest (and current funding) to report.
Figures
Fig. 1:. Cortical GABAergic microcircuit controlling stress vulnerability and resilience relevant for depressive disorders and antidepressant drug action.
The large majority of SST cells (i.e. >70%) represent Martinotti cells that preferentially innervate the distal apical tufts of pyramidal neurons (PN) were in cortical Layer 1 [105]. PV-positive interneurons preferentially innervate the somata, proximal dendrites, and axon initial segments of PNs and thereby exert powerful control over action potential firing of these neurons. VIP cells preferentially inhibit SST cells, which causes disinhibition of PNs. PV and SST cells also innervate each other. In particular, inhibition of PV cells by a subset of SST cells in layer 4 that are distinct from Martinotti cells, produces disinhibition of PNs [105]. The distinctive functional properties of SST cells suggest they exert a neuroprotective role. First, the activity of SST neurons, but not PV neurons in the prefrontal cortex scales with the strength of excitatory synaptic inputs from the basolateral amygdala (BLA) (1). Second, GABAergic inputs to PN dendrites buffer the temporal and spatial spread of NMDA receptor-mediated Ca2+ entry at adjacent glutamatergic synapses on dendritic spines [106] (2). Third, GABAergic inhibition of dendritic spines by SST neurons is principally mediated by postsynaptic α5-GABAARs, presumably composed of 2α5, 2β and 1γ2 subunit [92] (3). Fourth, genetically increasing the excitability of SST cells mimics the behavioral effects of antidepressant drug treatment [7] including stress resilience [8]. Lastly, SST neurons in the mPFC are direct targets also of excitatory inputs from the vHPC [71] (4).
Fig. 2:. Pathological and therapeutic roles of neurosteroids in the BLA in depression.
PV interneurons in the BLA are critical for governing the activity of BLA principal neurons and network states driving behavioral states. PV interneurons in the BLA uniquely express the δ-GABAARs. Chronic stress has been shown to decrease the expression of δ-GABAARs in the BLA, which is the primary site of action for neurosteroids, suggesting that neurosteroid signaling may be impaired in the BLA following chronic stress exposure. Consistent with deficits in neurosteroid signaling, chronic stress is associated with a reduction in the expression of 5α-reductase (Srd5a1/2), a key enzyme in neurosteroidogenesis. Further, the endogenous levels of allopregnanolone are decreased in the BLA following chronic stress. Given that chronic stress is a major risk factor for MDD and the evidence that neurosteroids exert robust anxiolytic and antidepressant effects, impaired endogenous neurosteroid signaling may contribute to deficits in emotional processing and MDD. Impaired endogenous neurosteroidogenesis related to MDD may also help explain the therapeutic efficacy of exogenous NAS treatment for restoring the healthy behavioral state and treating MDD.
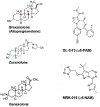
Fig 3:. Chemical formulas of neuroactive steroids and modulators of α5-GABAARs.
Left: Formulas of neuroactive steroids used in clinical studies of depressive disorders. Right: Formulas of modulators of the α5-GABAAR used in preclinical studies.
Fig. 4:. Model of a microcircuit relevant for cognition in depression and restoration of cognitive deficits with an α5-PAM.
A. Procognitive effects of α5-PAMs in depression through improved signal-to-noise ratio at the cellular level. Left panel: Dendrite-targeting somatostatin (SST)-positive interneurons inhibit pyramidal neurons via α5-GABAARs, resulting in a sparse baseline activity of the pyramidal neurons. This inhibition is mediated by α5-GABAAR (short green “B” arrows). Stronger excitatory signals are not inhibited by these SST interneurons (long green “S” arrows). This results in a high signal-to-noise ratio (SNR) which is required for optimal cognitive function. Middle panel: In depression, the excitatory activity of the pyramidal neurons is increased, while the inhibitory activity of the SST interneurons is decreased. The result is that the baseline activity of the pyramidal neurons is not as efficiently inhibited as in the healthy brain so that more baseline signals result in activation of the pyramidal neurons (B) and, while there is no change in the transmission of stronger excitatory signals through the pyramidal neurons (S), this results in a lower signal-to-noise-ratio with poor coding of information and cognitive deficits. Right panel: Administration of an α5-PAM strengthens the inhibition of the pyramidal neurons by the SST interneurons, so that incoming baseline activity is inhibited, as in the healthy condition, and not propagated, while stronger excitatory signals are propagated through the pyramidal neurons, resulting in restoration of a high signal-to-noise-ratio and normalized cognitive function. B. Cognition as a function of the activity of α5-GABAARs. There is an optimal level of activity of the α5-GABAAR system for cognitive performance. Both a lower and a higher activity result in cognitive impairments. In depression, the activity of the α5-GABAAR system is reduced, resulting in cognitive impairment. Administration of an α5-PAM increases the activity level of the α5-GABAAR system and thus restores learning and memory (L&M) performance.
Fig. 5 (KEY FIGURE):. Proposed mechanisms of α5-PAMs and α5-NAMs for normalization of defects in GABAergic and glutamatergic deficits in stress-associated psychiatric conditions.
Behavioral stress involves a rapid increase in glutamatergic synaptic transmission and acts over time to result in defects of GABAergic inhibition as evidenced by reduced expression of glutamic acid decarboxylase (GAD) and of the vesicular GABA transporter [107], reduced SST neuron function [5] [108], and reduced NAS synthesis [109]. Chronic stress and deficits in GABAergic synaptic inhibition are followed by homeostatic-like downregulation of AMPA and NMDA-type glutamate receptors and of the vesicular transporter for glutamate [101] [107]. Potentiation of GABAergic inhibition by PAMs appears to allow for a gradual recovery of glutamate receptor expression and eventually also of GABAergic transmission. Very different from GABAAR PAMs, acute doses of α5-GABAAR NAMs lead to a rapid and transient decrease of dendritic GABAergic inhibition, a corresponding increase in glutamatergic transmission and activity-induced and NMDAR-mediated plasticity including especially at α5-GABAAR expressing dendritic spines [92]. This mechanism of α5-GABAAR NAMs is reminiscent of the rapid antidepressant mechanism of subanesthetic ketamine, which involves selective and transient inhibition of PV and SST GABAergic interneurons followed by disinhibition of glutamatergic neurons and LTP-like NMDA receptor mediated potentiation of both GABAergic and glutamatergic synapses on pyramidal cells, lasting for days ([9] and reviewed in [10]).
Similar articles
- Understanding the mechanism of action and clinical effects of neuroactive steroids and GABAergic compounds in major depressive disorder.
Cutler AJ, Mattingly GW, Maletic V. Cutler AJ, et al. Transl Psychiatry. 2023 Jun 26;13(1):228. doi: 10.1038/s41398-023-02514-2. Transl Psychiatry. 2023. PMID: 37365161 Free PMC article. Review. - The Black Book of Psychotropic Dosing and Monitoring.
DeBattista C, Schatzberg AF. DeBattista C, et al. Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Review. - Altered γ-aminobutyric acid neurotransmission in major depressive disorder: a critical review of the supporting evidence and the influence of serotonergic antidepressants.
Pehrson AL, Sanchez C. Pehrson AL, et al. Drug Des Devel Ther. 2015 Jan 19;9:603-24. doi: 10.2147/DDDT.S62912. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25653499 Free PMC article. Review. - Development of neuroactive steroids for the treatment of postpartum depression.
Gunduz-Bruce H, Takahashi K, Huang MY. Gunduz-Bruce H, et al. J Neuroendocrinol. 2022 Feb;34(2):e13019. doi: 10.1111/jne.13019. Epub 2021 Aug 30. J Neuroendocrinol. 2022. PMID: 34462985 Free PMC article. Review. - A hypothesis of monoamine (5-HT) - Glutamate/GABA long neural circuit: Aiming for fast-onset antidepressant discovery.
Li YF. Li YF. Pharmacol Ther. 2020 Apr;208:107494. doi: 10.1016/j.pharmthera.2020.107494. Epub 2020 Jan 25. Pharmacol Ther. 2020. PMID: 31991195 Review.
Cited by
- Distinct mechanisms of allopregnanolone and diazepam underlie neuronal oscillations and differential antidepressant effect.
Takasu K, Yawata Y, Tashima R, Aritomi H, Shimada S, Onodera T, Taishi T, Ogawa K. Takasu K, et al. Front Cell Neurosci. 2024 Jan 8;17:1274459. doi: 10.3389/fncel.2023.1274459. eCollection 2023. Front Cell Neurosci. 2024. PMID: 38259500 Free PMC article. - Inhibitory Actions of Potentiating Neuroactive Steroids in the Human α1β3γ2L γ-Aminobutyric Acid Type A Receptor.
Pierce SR, Germann AL, Covey DF, Evers AS, Steinbach JH, Akk G. Pierce SR, et al. Mol Pharmacol. 2024 Oct 17;106(5):264-277. doi: 10.1124/molpharm.124.000960. Mol Pharmacol. 2024. PMID: 39214710 - Effects of chronic stress on cognitive function - From neurobiology to intervention.
Girotti M, Bulin SE, Carreno FR. Girotti M, et al. Neurobiol Stress. 2024 Sep 2;33:100670. doi: 10.1016/j.ynstr.2024.100670. eCollection 2024 Nov. Neurobiol Stress. 2024. PMID: 39295772 Free PMC article. Review. - Gut microbiota modulates depressive-like behaviors induced by chronic ethanol exposure through short-chain fatty acids.
Shen H, Zhang C, Zhang Q, Lv Q, Liu H, Yuan H, Wang C, Meng F, Guo Y, Pei J, Yu C, Tie J, Chen X, Yu H, Zhang G, Wang X. Shen H, et al. J Neuroinflammation. 2024 Nov 6;21(1):290. doi: 10.1186/s12974-024-03282-6. J Neuroinflammation. 2024. PMID: 39508236 Free PMC article. - Transcriptome signatures of the medial prefrontal cortex underlying GABAergic control of resilience to chronic stress exposure.
Shao M, Botvinov J, Banerjee D, Girirajan S, Lüscher B. Shao M, et al. Mol Psychiatry. 2024 Nov 16. doi: 10.1038/s41380-024-02832-x. Online ahead of print. Mol Psychiatry. 2024. PMID: 39550415
References
- Malhi GS and Mann JJ (2018) Depression. Lancet 392 (10161), 2299–2312. - PubMed
- Delgado PL (2000) Depression: the case for a monoamine deficiency. J Clin Psychiatry 61 Suppl 6, 7–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS102937/NS/NINDS NIH HHS/United States
- P50 MH122379/MH/NIMH NIH HHS/United States
- R01 GM128183/GM/NIGMS NIH HHS/United States
- R01 AA026256/AA/NIAAA NIH HHS/United States
- R01 MH128235/MH/NIMH NIH HHS/United States
- R01 NS105628/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous