New Devices in Glaucoma - PubMed (original) (raw)
Review
New Devices in Glaucoma
Lilian Chan et al. Ophthalmol Ther. 2023 Oct.
Abstract
Glaucoma remains a leading cause of blindness globally. Minimally invasive treatment techniques are rapidly expanding the availability of therapeutic options for glaucoma. These include devices aimed at enhancing outflow through the subconjunctival space, Schlemm's canal, and suprachoroidal space, sustained-release drug delivery devices, and extraocular devices aiming to reduce glaucomatous progression through other novel means. In this review, we provide an overview of several novel devices either newly available or in development for the medical and surgical management of glaucoma. Further studies are required to determine the long-term efficacy of these devices and how they will integrate into the current landscape of glaucoma management.
Keywords: Delayed-action preparation; Excimer laser trabeculostomy; Glaucoma; Glaucoma drainage implants; MINIject; Minimally invasive glaucoma surgery; Minimally invasive micro sclerostomy; Multi-pressure dial; Visiplate; iDOSE.
© 2023. The Author(s).
Conflict of interest statement
Lilian Chan and Qi N Cui have nothing to disclose. Marlene R Moster: Abbvie: Consultant/Advisor, Lecture Fees/Speakers Bureau, Grant Support. ArcScan: Advisory Board. AeriePharmaceuticals: Consultant/Advisor, Lecture Fees/Speakers Bureau, Grant Support. AlconLaboratories, Inc.: Consultant/Advisor, Lecture Fees/Speakers Bureau, Grant Support. Allergan: Consultant/Advisor, Lecture Fees/Speakers Bureau, Grant Support. Bausch + Lomb: Lecture Fees/Speakers Bureau, Grant Support. Glaukos: Grant Support. InnFocus: Grant Support. Iridex: Lecture Fees, Grant Support. MedEdicus: Lecture Fees/Speakers Bureau. NewWorldMedical, Inc.: Grant Support. Nicox: Grant Support Wills Eye. Novartis, AlconPharmaceuticals: Lecture Fees/Speakers Bureau. Regeneron: Advisor. Santen, Inc.: Consultant/Advisor, Grant Support. Thea Inc.: Grant Support, Advisor. W. L. Gore & Associates, Inc.: Advisor. Amanda Bicket: W.L. Gore & Associates: Consultant. Arsham Sheybani: Alcon, Abbvie, Nova Eye, and Glaukos: Consultant. Steven R Sarkisian: Alcon Laboratories, Inc.: Consultant/Advisor, Lecture Fees/Speakers Bureau, Grant Support. Allergan, Inc.: Consultant/Advisor, Lecture Fees/Speakers Bureau, Grant Support. Allysta Pharmaceuticals: Grant Support. Bausch + Lomb: Consultant/Advisor, Lecture Fees/Speakers Bureau. Beaver-Visitec International, Inc.: Consultant/Advisor. Elios: Grant Support. Glaukos Corporation: Consultant/Advisor, Grant Support. Icare USA, Inc.: Consultant/Advisor. iSTAR Medical: Grant Support. Katena Products, Inc: Consultant/Advisor. MicroSurgical Technology: Consultant/Advisor. Ocular Science: Consultant/Advisor, Grant Support, Stock Options—Public or Private Corp. Ocular Therapeutix: Grant Support. Santen, Inc.: Consultant/Advisor. Sight Sciences, Inc.: Consultant/Advisor, Grant Support, Equity/Stock Holder—Public Corp. Thomas W Samuelson: Equinox: consultant, advisory board member. Iqbal Ike K Ahmed: Aequus: Consultant/Consulting Fees. Ace Vision: Consultant/Consulting Fees. Aerie Pharmaceuticals: Consultant/Consulting Fees, Research Grant/Support. Akorn: Consultant/Consulting Fees. Alcon: Consultant/Consulting Fees,Speakers Honoraria, Research Grant/Support. Allergan: Consultant/Consulting Fees,Speakers Honoraria, Research Grant/Support. Aquea Health, Inc: Consultant/Consulting Fees. ArcScan: Consultant/Consulting Fees. Avellino Lab USA, Inc: Consultant/Consulting Fees. Avisi: Consultant/Consulting Fees. Bausch Health: Consultant/Consulting Fees. Beaver Visitec: Consultant/Consulting Fees. Belkin Vision: Consultant/Consulting Fees. Beyeonics: Consultant/Consulting Fees. Bionode: Consultant/Consulting Fees, Research Grant/Support. Carl Zeiss Meditec: Consultant/Consulting Fees, Speakers Honoraria. Centricity Vision, Inc: Consultant/Consulting Fees. CorNeat Vision: Consultant/Consulting Fees. Custom Surgical: Consultant/Consulting Fees. Elios Vision: Consultant/Consulting Fees. ElutiMed: Consultant/Consulting Fees. Equinox: Consultant/Consulting Fees. EyeFlow, Inc: Consultant/Consulting Fees. EyedMed: Consultant/Consulting Fees. EyeQ Technologies: Consultant/Consulting Fees. Exhaura Limited: Consultant/Consulting Fees. Genentech: Consultant/Consulting Fees. Glaukos: Consultant/Consulting Fees, Research Grant/Support. Gore: Consultant/Consulting Fees. Heine: Consultant/Consulting Fees, Speakers Honoraria. Heru: Consultant/Consulting Fees. Hexiris Pharma: Consultant/Consulting Fees. Iantrek: Consultant/Consulting Fees. InjectSense: Consultant/Consulting Fees. Iridex: Consultant/Consulting Fees. iCare: Research Grant/Support. iStar: Consultant/Consulting Fees. Ivantis: Consultant/Consulting Fees, Research Grant/Support. Johnson & Johnson Vision: Consultant/Consulting Fees, Speakers Honoraria, Research Grant/Support. Labtician Thea: Consultant/Consulting Fees. LayerBio: Consultant/Consulting Fees. Leica Microsystems: Consultant/Consulting Fees. Life Long Vision: Consultant/Consulting Fees. Long Bridge Medical, Inc: Consultant/Consulting Fees. MicroOptx: Consultant/Consulting Fees. MST Surgical: Consultant/Consulting Fees, Speakers Honoraria. Myra Vision/Shifamed LLC: Consultant/Consulting Fees. New World Medical: Consultant/Consulting Fees, Research Grant/Support. NovaEye: Consultant/Consulting Fees. Ocular Instruments: Consultant/Consulting Fees. Ocular Therapeutix: Consultant/Consulting Fees. Oculo: Consultant/Consulting Fees. Oculus Surgical: Consultant/Consulting Fees. Omega Ophthalmics: Consultant/Consulting Fees. PolyActiva: Consultant/Consulting Fees. PulseMedica: Consultant/Consulting Fees. Radiance Therapeutics, Inc: Consultant/Consulting Fees. Radius XR: Consultant/Consulting Fees. Rheon Medical SA: Consultant/Consulting Fees. Ripple Therapeutics: Consultant/Consulting Fees. Samsara Vision: Consultant/Consulting Fees. Sanoculis: Consultant/Consulting Fees. Santen: Consultant/Consulting Fees, Research Grant/Support. Singapore Biodesign Programme Office. Sight Sciences: Consultant/Consulting Fees. Smartlens, Inc: Consultant/Consulting Fees. Stroma: Consultant/Consulting Fees. Thea Pharma: Consultant/Consulting Fees. TFS Health Science: Consultant/Consulting Fees. ViaLase: Consultant/Consulting Fees. Visus Therapeutics: Consultant/Consulting Fees. Vizzario: Consultant/Consulting Fees. VSY Biotechnology: Consultant/Consulting Fees. Zilia, Inc: Consultant/Consulting Fees. Eydie Miller-Ellis: Avisi: scientific advisor. Aerie Pharmaceuticals, Allergan, Eyenovia, and Thea Pharama: consultant. Thea Pharma and NIH/NEI: research support. Oluwatosin U Smith: Alcon, Iridex, Santen, New World Medical, Glaukos, and Iris Vision: consultant. Aerie, Bauch and Lomb, and Allergan: speaker/consultant. Nova Eye Medical and Olleyes: stock holder. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the authors.
Figures
Fig. 1
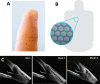
Visiplate aqueous shunt [73]. A Image of the device (balanced on the finger tip) demonstrating its profile. B The material consists of microchannels intended to create multiple outflow pathways while maintaining resistance to prevent hypotony. C Serial optical coherence tomography images of an implant in a human eye. The device remained in position through month 3, extending from the anterior chamber to the subconjunctival space with a shallow bleb overlying the plate
Fig. 2
GORE glaucoma drainage implant (GDI) concept [12]. A Current design of the GORE GDI, with a tube connected to an inflatable reservoir composed of proprietary bilayered GORE ePTFE. B Electron microscopy of a sheet of the bilayered ePTFE. The external facing surface is designed to facilitate tissue ingrowth and is backed by an aqueous permeable cell exclusion layer. C Two sheets of the bilayered material are joined and sealed to form a pocket. Aqueous is shunted into the reservoir via the tube and then percolates through the GORE material into the surrounding tissues
Fig. 3
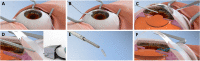
Minimally invasive micro-sclerostomy (MIMS) procedure [74, 75]. A Mitomycin C and viscoelastic are delivered to the subconjunctival space to the area where aqueous will be shunted via the sclerotomy. B A side port is created, and the the anterior chamber is filled with viscoelastic. C The MIMS surgical device is inserted ab interno and advanced above the iris plane towards the angle. D, E The MIMS footpedal is used to create the drainage channel, and a cylinder of scleral tissue is removed, F allowing for flow from the aqueous chamber to the subconjunctival space
Fig. 4
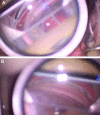
Excimer laser trabeculostomy [73]. A A probe applies laser energy directly to the trabecular meshwork using a foot pedal system. B Blood and microbubbles are often seen post-intervention
Fig. 5
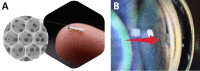
iSTAR MINIject [73]. A The device is 5 mm × 1 mm and made of proprietary silicone material. B Final placement of device in the suprachoroidal space (red arrow). Proper placement is achieved when the green ring is at the level of scleral spur
Fig. 6
Iantrek biostent [73]. A The implant is inserted into a cyclodialysis cannula for ab interno delivery. B Once deployed, the biostent reinforces an iatrogenic cyclodialysis cleft, allowing for drainage around and through the porous device into the suprachoroidal space
Fig. 7
iDose implant [73]. A The device is anchored into the scleral tissue behind the trabecular meshwork. B Image of the implant in the anterior chamber angle nasally in a human eye.
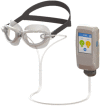
Fig. 8
Multi-pressure dial (MPD) [73]. The device consists of airtight goggles attached to a titratable negative pressure pump
Similar articles
- Advances in Excimer Laser Trabeculostomy within the Landscape of Minimally-Invasive Glaucoma Surgery.
Nguyen A, Simon B, Doan R, Chen E, Lamrani R, Shakibkhou J, Berlin MS. Nguyen A, et al. J Clin Med. 2022 Jun 17;11(12):3492. doi: 10.3390/jcm11123492. J Clin Med. 2022. PMID: 35743562 Free PMC article. - Microfluidics in the eye: a review of glaucoma implants from an engineering perspective.
Fang Z, Bi S, Brown JD, Chen J, Pan T. Fang Z, et al. Lab Chip. 2023 Nov 7;23(22):4736-4772. doi: 10.1039/d3lc00407d. Lab Chip. 2023. PMID: 37847237 Review. - Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents.
Pillunat LE, Erb C, Jünemann AG, Kimmich F. Pillunat LE, et al. Clin Ophthalmol. 2017 Aug 29;11:1583-1600. doi: 10.2147/OPTH.S135316. eCollection 2017. Clin Ophthalmol. 2017. PMID: 28919702 Free PMC article. - Minimally invasive glaucoma surgery: current status and future prospects.
Richter GM, Coleman AL. Richter GM, et al. Clin Ophthalmol. 2016 Jan 28;10:189-206. doi: 10.2147/OPTH.S80490. eCollection 2016. Clin Ophthalmol. 2016. PMID: 26869753 Free PMC article. Review. - Subconjunctival draining minimally-invasive glaucoma devices for medically uncontrolled glaucoma.
King AJ, Shah A, Nikita E, Hu K, Mulvaney CA, Stead R, Azuara-Blanco A. King AJ, et al. Cochrane Database Syst Rev. 2018 Dec 16;12(12):CD012742. doi: 10.1002/14651858.CD012742.pub2. Cochrane Database Syst Rev. 2018. PMID: 30554418 Free PMC article.
Cited by
- One-Year Comparison of Efficacy and Safety of PreserFlo MicroShunt with Mitomycin C Applied by Sub-Tenon Injection Versus Sponge.
Majtanova N, Takacova A, Kurilova V, Hejsek L, Majtan J, Kolar P. Majtanova N, et al. Ophthalmol Ther. 2025 Jan;14(1):153-167. doi: 10.1007/s40123-024-01074-y. Epub 2024 Nov 22. Ophthalmol Ther. 2025. PMID: 39576486 Free PMC article. - Historical and Contemporary Debates in Schlemm's Canal-Based MIGS.
Chihara E, Hamanaka T. Chihara E, et al. J Clin Med. 2024 Aug 19;13(16):4882. doi: 10.3390/jcm13164882. J Clin Med. 2024. PMID: 39201024 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources