Vaccines for preventing herpes zoster in older adults - PubMed (original) (raw)
Review
Vaccines for preventing herpes zoster in older adults
Juliana de Oliveira Gomes et al. Cochrane Database Syst Rev. 2023.
Abstract
Background: Herpes zoster, commonly known as shingles, is a neurocutaneous disease caused by the reactivation of the virus that causes varicella (chickenpox). After resolution of the varicella episode, the virus can remain latent in the sensitive dorsal ganglia of the spine. Years later, with declining immunity, the varicella zoster virus (VZV) can reactivate and cause herpes zoster, an extremely painful condition that can last many weeks or months and significantly compromise the quality of life of the affected person. The natural process of ageing is associated with a reduction in cellular immunity, and this predisposes older adults to herpes zoster. Vaccination with an attenuated form of the VZV activates specific T-cell production avoiding viral reactivation. Two types of herpes zoster vaccines are currently available. One of them is the single-dose live attenuated zoster vaccine (LZV), which contains the same live attenuated virus used in the chickenpox vaccine, but it has over 14-fold more plaque-forming units of the attenuated virus per dose. The other is the recombinant zoster vaccine (RZV) which does not contain the live attenuated virus, but rather a small fraction of the virus that cannot replicate but can boost immunogenicity. The recommended schedule for the RZV is two doses two months apart. This is an update of a Cochrane Review first published in 2010, and updated in 2012, 2016, and 2019.
Objectives: To evaluate the effectiveness and safety of vaccination for preventing herpes zoster in older adults.
Search methods: For this 2022 update, we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2022, Issue 10), MEDLINE (1948 to October 2022), Embase (2010 to October 2022), CINAHL (1981 to October 2022), LILACS (1982 to October 2022), and three trial registries.
Selection criteria: We included studies involving healthy older adults (mean age 60 years or older). We included randomised controlled trials (RCTs) or quasi-RCTs comparing zoster vaccine (any dose and potency) versus any other type of intervention (e.g. varicella vaccine, antiviral medication), placebo, or no intervention (no vaccine). Outcomes were cumulative incidence of herpes zoster, adverse events (death, serious adverse events, systemic reactions, or local reaction occurring at any time after vaccination), and dropouts.
Data collection and analysis: We used the standard methodological procedures expected by Cochrane.
Main results: We included two new studies involving 1736 participants in this update. The review now includes a total of 26 studies involving 90,259 healthy older adults with a mean age of 63.7 years. Only three studies assessed the cumulative incidence of herpes zoster in groups that received vaccines versus placebo. Most studies were conducted in high-income countries in Europe and North America and included healthy Caucasians (understood to be white participants) aged 60 years or over with no immunosuppressive comorbidities. Two studies were conducted in Japan and one study was conducted in the Republic of Korea. Sixteen studies used LZV. Ten studies tested an RZV. The overall certainty of the evidence was moderate, which indicates that the intervention probably works. Most data for the primary outcome (cumulative incidence of herpes zoster) and secondary outcomes (adverse events and dropouts) came from studies that had a low risk of bias and included a large number of participants. The cumulative incidence of herpes zoster at up to three years of follow-up was lower in participants who received the LZV (one dose subcutaneously) than in those who received placebo (risk ratio (RR) 0.49, 95% confidence interval (CI) 0.43 to 0.56; risk difference (RD) 2%; number needed to treat for an additional beneficial outcome (NNTB) 50; moderate-certainty evidence) in the largest study, which included 38,546 participants. There were no differences between the vaccinated and placebo groups for serious adverse events (RR 1.08, 95% CI 0.95 to 1.21) or deaths (RR 1.01, 95% CI 0.92 to 1.11; moderate-certainty evidence). The vaccinated group had a higher cumulative incidence of one or more adverse events (RR 1.71, 95% CI 1.38 to 2.11; RD 23%; number needed to treat for an additional harmful outcome (NNTH) 4.3) and injection site adverse events (RR 3.73, 95% CI 1.93 to 7.21; RD 28%; NNTH 3.6; moderate-certainty evidence) of mild to moderate intensity. These data came from four studies with 6980 participants aged 60 years or older. Two studies (29,311 participants for safety evaluation and 22,022 participants for efficacy evaluation) compared RZV (two doses intramuscularly, two months apart) versus placebo. Participants who received the new vaccine had a lower cumulative incidence of herpes zoster at 3.2 years follow-up (RR 0.08, 95% CI 0.03 to 0.23; RD 3%; NNTB 33; moderate-certainty evidence), probably indicating a favourable profile of the intervention. There were no differences between the vaccinated and placebo groups in cumulative incidence of serious adverse events (RR 0.97, 95% CI 0.91 to 1.03) or deaths (RR 0.94, 95% CI 0.84 to 1.04; moderate-certainty evidence). The vaccinated group had a higher cumulative incidence of adverse events, any systemic symptom (RR 2.23, 95% CI 2.12 to 2.34; RD 33%; NNTH 3.0), and any local symptom (RR 6.89, 95% CI 6.37 to 7.45; RD 67%; NNTH 1.5). Although most participants reported that their symptoms were of mild to moderate intensity, the risk of dropouts (participants not returning for the second dose, two months after the first dose) was higher in the vaccine group than in the placebo group (RR 1.25, 95% CI 1.13 to 1.39; RD 1%; NNTH 100, moderate-certainty evidence). Only one study reported funding from a non-commercial source (a university research foundation). All other included studies received funding from pharmaceutical companies. We did not conduct subgroup and sensitivity analyses AUTHORS' CONCLUSIONS: LZV (single dose) and RZV (two doses) are probably effective in preventing shingles disease for at least three years. To date, there are no data to recommend revaccination after receiving the basic schedule for each type of vaccine. Both vaccines produce systemic and injection site adverse events of mild to moderate intensity. The conclusions did not change in relation to the previous version of the systematic review.
Trial registration: ClinicalTrials.gov NCT03120364 NCT00886613 NCT01505647 NCT01777321 NCT03894969 NCT04334577 NCT02180295 NCT02526745 NCT03116594 NCT04091451 NCT04210752 NCT04869982 NCT05007041 NCT05047770 NCT05219253 NCT05245838.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Anna MZ Gagliardi has declared that they have no conflict of interest. Brenda NG Andriolo has declared that they have no conflict of interest. Maria R Torloni has declared that they have no conflict of interest. Juliana O Gomes has declared that they have no conflict of interest. Regis B Andriolo has declared that they have no conflict of interest. Eduardo C Cruz has declared that they have no conflict of interest.
Figures
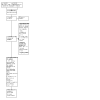
1
Study flow diagram 2022 update.
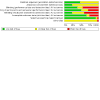
2
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
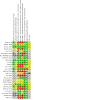
3
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
1.1. Analysis
Comparison 1: Live zoster vaccine versus placebo, Outcome 1: Cumulative incidence of herpes zoster
1.2. Analysis
Comparison 1: Live zoster vaccine versus placebo, Outcome 2: Participants with adverse events
1.3. Analysis
Comparison 1: Live zoster vaccine versus placebo, Outcome 3: Duration in days of adverse events
1.4. Analysis
Comparison 1: Live zoster vaccine versus placebo, Outcome 4: Dropouts
1.5. Analysis
Comparison 1: Live zoster vaccine versus placebo, Outcome 5: Participants with no follow‐up
2.1. Analysis
Comparison 2: Recombinant zoster vaccine versus placebo, Outcome 1: Cumulative incidence of herpes zoster at least 3.2 years follow‐up
2.2. Analysis
Comparison 2: Recombinant zoster vaccine versus placebo, Outcome 2: Incidence of herpes zoster at least 4 years follow‐up
2.3. Analysis
Comparison 2: Recombinant zoster vaccine versus placebo, Outcome 3: Participants with adverse events
2.4. Analysis
Comparison 2: Recombinant zoster vaccine versus placebo, Outcome 4: Dropouts
Update of
- Vaccines for preventing herpes zoster in older adults.
Gagliardi AM, Andriolo BN, Torloni MR, Soares BG, de Oliveira Gomes J, Andriolo RB, Canteiro Cruz E. Gagliardi AM, et al. Cochrane Database Syst Rev. 2019 Nov 7;2019(11):CD008858. doi: 10.1002/14651858.CD008858.pub4. Cochrane Database Syst Rev. 2019. PMID: 31696946 Free PMC article. Updated.
Similar articles
- Vaccines for preventing herpes zoster in older adults.
Gagliardi AM, Andriolo BN, Torloni MR, Soares BG, de Oliveira Gomes J, Andriolo RB, Canteiro Cruz E. Gagliardi AM, et al. Cochrane Database Syst Rev. 2019 Nov 7;2019(11):CD008858. doi: 10.1002/14651858.CD008858.pub4. Cochrane Database Syst Rev. 2019. PMID: 31696946 Free PMC article. Updated. - Vaccines for preventing herpes zoster in older adults.
Gagliardi AM, Andriolo BN, Torloni MR, Soares BG. Gagliardi AM, et al. Cochrane Database Syst Rev. 2016 Mar 3;3(3):CD008858. doi: 10.1002/14651858.CD008858.pub3. Cochrane Database Syst Rev. 2016. PMID: 26937872 Free PMC article. Updated. Review. - Vaccines for preventing herpes zoster in older adults.
Gagliardi AM, Gomes Silva BN, Torloni MR, Soares BG. Gagliardi AM, et al. Cochrane Database Syst Rev. 2012 Oct 17;10:CD008858. doi: 10.1002/14651858.CD008858.pub2. Cochrane Database Syst Rev. 2012. PMID: 23076951 Updated. Review. - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - Vaccines for preventing typhoid fever.
Milligan R, Paul M, Richardson M, Neuberger A. Milligan R, et al. Cochrane Database Syst Rev. 2018 May 31;5(5):CD001261. doi: 10.1002/14651858.CD001261.pub4. Cochrane Database Syst Rev. 2018. PMID: 29851031 Free PMC article. Review.
Cited by
- Safety of Adjuvanted Recombinant Herpes Zoster Virus Vaccination in Fragile Populations: An Observational Real-Life Study.
Costantino M, Giudice V, Moccia G, Longanella W, Caruccio S, Tremiterra G, Sinopoli P, Benvenuto D, Serio B, Malatesta F, Pecoraro N, Vozzella EA, Rossiello R, Genovese G, De Caro F. Costantino M, et al. Vaccines (Basel). 2024 Aug 29;12(9):990. doi: 10.3390/vaccines12090990. Vaccines (Basel). 2024. PMID: 39340022 Free PMC article. - Humoral Cytokine Levels in Patients with Herpes Zoster: A Meta-Analysis.
Yue J, Yao M. Yue J, et al. J Pain Res. 2024 Mar 5;17:887-902. doi: 10.2147/JPR.S449211. eCollection 2024. J Pain Res. 2024. PMID: 38476878 Free PMC article. Review. - Immunogenicity and safety of live attenuated and recombinant/inactivated varicella zoster vaccines in people living with HIV: A systematic review.
Carta V, Mangeri L, Tiecco G, Focà E, Quiros-Roldan E, De Francesco MA. Carta V, et al. Hum Vaccin Immunother. 2024 Dec 31;20(1):2341456. doi: 10.1080/21645515.2024.2341456. Epub 2024 Apr 22. Hum Vaccin Immunother. 2024. PMID: 38650460 Free PMC article. Review. - The Burden of Herpes Zoster on Hospital Admissions: A Retrospective Analysis in the Years of 2015-2021 from the Abruzzo Region, Italy.
Scampoli P, Di Martino G, Cedrone F, Odio C, Di Giovanni P, Romano F, Staniscia T. Scampoli P, et al. Vaccines (Basel). 2024 Apr 26;12(5):462. doi: 10.3390/vaccines12050462. Vaccines (Basel). 2024. PMID: 38793713 Free PMC article. - Genetic insights into the gut microbiota, herpes zoster, and postherpetic neuralgia: a bidirectional two-sample Mendelian randomization study.
Deng Z, Liu Y, Wang H, Luo T. Deng Z, et al. Front Genet. 2024 May 23;15:1366824. doi: 10.3389/fgene.2024.1366824. eCollection 2024. Front Genet. 2024. PMID: 38846958 Free PMC article.
References
References to studies included in this review
Beals 2016 {published data only}
- Beals CR, Railkar RA, Schaeffer AK, Levin Y, Kochba E, Meyer BK, et al. Immune response and reactogenicity of intradermal administration versus subcutaneous administration of varicella-zoster virus vaccine: an exploratory, randomised, partly blinded trial. Lancet Infectious Diseases 2016;16(8):915-22. - PubMed
Berger 1998 {published data only}
- Berger R, Trannoy E, Holländer G, Bailleux F, Rudin C, Creusvaux H. A dose-response study of a live attenuated varicella-zoster virus (Oka strain) vaccine administered to adults 55 years of age and older. Journal of Infectious Diseases 1998;178(Suppl 1):99-103. [0022-1899/98/78S1-0022$02.00] - PubMed
- Trannoy E, Berger R, Holländer G, Bailleux F, Heimendinger P, Vuillier D, et al. Vaccination of immunocompetent elderly subjects with a live attenuated Oka strain of varicella zoster virus: a randomized, controlled, dose-response trial. Vaccine 2000;18(16):1700-6. [PII: S0264-410X (99) 00510-1] - PubMed
Chlibek 2013 {published data only (unpublished sought but not used)}
- Chlibek R, Bayas JM, Collins H, Pinta MLR, Ledent E, Johann F, et al. Safety and immunogenicity of an AS01 adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults ≥ 50 years of age. Journal of Infectious Diseases 2013;208:1953–61. - PubMed
Chlibek 2014 {published data only (unpublished sought but not used)}
- Chlibek R, Smetana J, Pauksens K, Rombo L, Van den Hoek JA, Richardus JH, et al. Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: a phase II, randomized, controlled study. Vaccine 2014;32(15):1745-53. - PubMed
Choi 2019 {published data only}
- Choi WS, Choi JH, Jung DS, Choi HJ, Kim YS, Lee J, et al. Immunogenicity and safety of a new live attenuated herpes zoster vaccine (NBP608) compared to Zostavax in healthy adults aged 50 years and older. Vaccine 2019;37(27):3605–10. - PubMed
Cunningham 2016 {published data only}
- Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Díez-Domingo J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. New England Journal of Medicine 2016;375(11):1019-32. - PubMed
- Ikematsu H, Yamashita N, Ogawa M, Hirano M, Kovac M, Watanabe D. Efficacy, safety and immunogenicity of new adjuvanted herpes zoster subunit vaccine for Japanese over 50 years old and over 70 years old. Kansenshogaku Zasshi 2018;92(2):103-14.
Diez‐Domingo 2015 {published and unpublished data}
- Diez-Domingo J, Weinke T, Garcia de Lomas J, Meyer CU, Bertrand I, Eymin C, et al. Comparison of intramuscular and subcutaneous administration of a herpes zoster live-attenuated vaccine in adults aged ≥ 50 years: a randomised non-inferiority clinical trial. Vaccine 2015;33(6):789-95. [MEDLINE: ] - PubMed
- Diez-Domingo J, Weinke T, Kieninger-Baum D, Eymin C, Thomas S, Sadorged C. A clinical study of a shingles (herpes zoster) vaccine (live) administered by intramuscular or subcutaneous routes in adults aged ≥ 50 years. European Geriatric Medicine 2013;4(Suppl):81–141.
Gilderman 2008 {published data only}
- Gilderman LI, Lawless JF, Nolen TM, Sterling T, Rutledge RZ, Fernsler DA, et al. A double-blind, randomized, controlled, multicenter safety and immunogenicity study of a refrigerator-stable formulation of Zostavax. Clinical and Vaccine Immunology 2008;15(2):314-9. [DOI: 10.1128/CVI.00310-07] - DOI - PMC - PubMed
Hata 2016 {published data only}
Lal 2015 {published data only}
Lal 2018 {published data only}
- Lal H, Poder A, Campora L, Geeraerts B, Oostvogels L, Abeele CV, et al. Immunogenicity, reactogenicity and safety of 2 doses of an adjuvanted herpes zoster subunit vaccine administered 2, 6 or 12 months apart in older adults: results of a phase III, randomized, open-label, multicenter study. Vaccine 2018;36(1):148-54. [DOI: 10.1016/j.vaccine.2017.11.019] - DOI - PubMed
Levin 2000 {published and unpublished data}
- Levin MJ, Ellison MC, Zerbe GO, Barber D, Chan C, Stinson D, et al. Comparison of a live attenuated and an inactivated varicella vaccine to boost the varicella-specific immune response in seropositive people 55 years of age and older. Vaccine 2000;18(25):2915-20. [PMID: ] - PubMed
Levin 2018 {published data only}
Maréchal 2018 {published data only}
- Maréchal C, Lal H, Poder A, Ferguson M, Enweonye I, Heineman TC, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine co-administered with the 23-valent pneumococcal polysaccharide vaccine in adults ≥ 50 years of age: randomized trial. Vaccine 2018;36(29):4278-86. [DOI: 10.1016/j.vaccine.2018.05.110] - DOI - PubMed
Mills 2010 {published data only}
Min 2022 {published data only}
- Min JY, Mwakingwe-Omari A, Riley M, Molo LY, Soni J, Girard G, et al. The adjuvanted recombinant zoster vaccine co-administered with the 13-valent pneumococcal conjugate vaccine in adults aged ≥50 years: a randomized trial. Journal of Infection 2022;84:490-8. - PubMed
Murray 2011 {published data only}
NCT00886613 {published data only}
- NCT00886613. A study to evaluate immunity to varicella zoster virus after immunization with V212 vaccine or zostavax (V212-003) [A double-blind, randomized, placebo controlled, parallel group study to evaluate biomarkers of immunity to varicella zoster virus following immunization with V212/heat-treated varicella-zoster virus (VZV) vaccine or with ZOSTAVAX in healthy volunteers]. clinicaltrials.gov/ct2/show/NCT00886613 (first received 22 April 2009).
NCT01505647 {published data only}
- NCT01505647. Safety and immunogenicity of zoster vaccine (ZOSTAVAX™) made with an alternative manufacturing process (AMP) (V211-042 AM1) [A phase III double-blinded, randomized, multicenter, controlled study to evaluate the safety, tolerability, and immunogenicity of ZOSTAVAX™ made with an alternative manufacturing process (AMP)]. clinicaltrials.gov/ct2/show/study/NCT01505647 (first received 4 January 2012).
Oxman 2005 {published data only}
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. New England Journal of Medicine 2005;352(22):2271-84. [PMID: ] - PubMed
- Schmader KE, Johnson GR, Saddier P, Ciarleglio M, Wang WWB, Zhang JH, et al. Effect of a zoster vaccine on herpes zoster-related interference with functional status and health-related quality-of-life measures in older adults. Journal of the American Geriatrics Society 2010;58(9):1634-41. [DOI: 10.1111/j.1532-5415.2010.03021.x] - DOI - PMC - PubMed
- Simberkoff MS, Arbeit RD, Johnson GR, Oxman MN, Boardman KD, Williams HM, et al. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Annals of Internal Medicine 2010;152(9):545-54. [PMID: ] - PubMed
Schwarz 2017 {published data only}
- Schwarz TF, Aggarwal N, Moeckesch B, Schenkenberger I, Claeys C, Douha M, et al. Immunogenicity and safety of an adjuvanted herpes zoster subunit vaccine co-administered with seasonal influenza vaccine in adults aged 50 years or older. Journal of Infectious Diseases 2017;216(11):1352-61. [DOI: 10.1093/infdis/jix481] - DOI - PMC - PubMed
Strezova 2019 {published data only}
- Strezova A, Lal H, Enweonye I, Campora L, Beukelaers P, Segall N, et al. The adjuvanted recombinant zoster vaccine co-administered with a tetanus, diphtheria and pertussis vaccine in adults aged >50 years: a randomized trial [Study to assess the immunogenicity and safety of GlaxoSmithKline (GSK) Biologicals' herpes zoster subunit (HZ/su) vaccine (GSK1437173A) when co-administered with GSK Biologicals' diphtheria, tetanus and pertussis vaccine (Boostrix®) in adults aged 50 years and older]. Vaccine 2019;37(39):5877–85. - PubMed
Tyring 2007 {published data only}
Vermeulen 2012 {published data only}
Vesikari 2013 {published data only (unpublished sought but not used)}
- Vesikari T, Hardt R, Rümke HC, Icardi G, Montero J, Thomas S, et al. Immunogenicity and safety of a live attenuated shingles (herpes zoster) vaccine (Zostavax®) in individuals aged ≥ 70 years. A randomized study of a single dose vs. two different two-dose schedules. Human Vaccines and Immunotherapeutics 2013;9(4):1–7. - PMC - PubMed
Vink 2017 {published data only}
- Vink P, Shiramoto M, Ogawa M, Eda M, Douha M, Heineman T. Safety and immunogenicity of a herpes zoster subunit vaccine in Japanese population aged ≥ 50 years when administered subcutaneously vs. intramuscularly. Human Vaccines and Immunotherapeutics 2017;13(3):574-8. [DOI: 10.1080/21645515.2016.1232787] - DOI - PMC - PubMed
References to studies excluded from this review
Hayward 1994 {published data only}
- Hayward AR, Buda K, Levin MJ. Immune response to secondary immunization with live or inactivated VZV vaccine in elderly adults. Viral Immunology 1994;7(1):31-6. [PMID: ] - PubMed
Hayward 1996 {published data only}
- Hayward AR, Buda K, Jones M, White CJ, Levin MJ. Varicella zoster virus specific cytotoxicity following secondary immunization with live or killed vaccine. Viral Immunology 1996;9(4):241-5. [PMID: ] - PubMed
Irwin 2007 {published data only}
Johnson 2022 {published data only}
Kerzner 2007 {published data only}
- Kerzner B, Murray AV, Gheng E, Ifle R, Harvey PR, Tomlinson M, et al. Safety and immunogenicity profile of the concomitant administration of ZOSTAVAX and inactivated influenza vaccine in adults aged 50 and older. Journal of the American Geriatrics Society 2007;55(10):1499-507. [DOI: 10.1111/j.1532-5415.2007.01397.x] - DOI - PubMed
Kovac 2018 {published data only}
- Kovac M, Lal H, Cunningham AL, Levin MJ, Johnson RW, Campora L, et al, ZOE-50/70 Study Group. Complications of herpes zoster in immunocompetent older adults: incidence in vaccine and placebo groups in two large phase 3 trials. Vaccine 2018;36(12):1537-41. [DOI: 10.1016/j.vaccine.2018.02.029] - DOI - PubMed
Leroux‐Roels 2012 {published data only}
- Leroux-Roels I, Leroux-Roels G, Frédéric Clement F, Vandepapelière P, Vassilev V, Ledent E, et al. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein E subunit vaccine candidate in young and older adults. Journal of Infectious Diseases 2012;206(8):1280-90. [DOI: 10.1093/infdis/jis497] - DOI - PubMed
Macaladad 2007 {published data only}
MacIntyre 2010 {published data only}
- MacIntyre CR, Egerton T, McCaughey M, Parrino J, Campbell BV, Su SC, et al. Concomitant administration of zoster and pneumococcal vaccines in adults ≥ 60 years old. Human Vaccines 2010;6(11):894-902. [PMID: ] - PubMed
NCT03894969 {unpublished data only}2018‐002977‐24
- Investigators: Study Director - GSK Clinical Trials - GlaxoSmithKline. Immunogenicity and safety study of GSK's investigational vaccine (GSK3277511A) when administered in healthy smokers and ex-smokers following receipt of shingrix vaccine. clinicaltrials.gov 2022. [CLINICALTRILAS.GOV: NCT03894969]
Patterson‐Bartlett 2007 {published data only}
- Patterson-Bartlett J, Levin MJ, Lang N, Schödel FP, Vessey R, Weingerg A. Phenotypic and functional characterization of ex vivo T cell responses to the live attenuated herpes zoster vaccine. Vaccine 2007;25(41):7087-93. [PMID: ] - PubMed
Strezova 2017 {published data only}
Weinberg 2018 {published data only}
References to studies awaiting assessment
NCT03314103 {unpublished data only}
- NCT03314103. A multi-center, randomized, double-blinded, phase 3 trial to evaluate the efficacy against herpes zoster of a live attenuated varicella-zoster virus vaccine in adults over 40 years of age. clinicaltrials.gov/ct2/show/NCT03314103 (first received 19 October 2017).
NCT04334577 {unpublished data only}
- NCT04334577. Evaluation of the efficacy of attenuated zoster vaccine, live from herpes zoster in adults aged 40 years or older a multicenter, randomized, double-blinded, placebo-controlled trial phase III. clinicaltrials.gov/ct2/show/NCT04334577 (first received 6 April 2020).
References to ongoing studies
NCT02180295 {unpublished data only}
- NCT02180295. A lot-to-lot consistency study to evaluate safety, tolerability, and immunogenicity of inactivated varicella zoster virus (VZV) vaccine in healthy adults (V212-014). clinicaltrials.gov/ct2/show/NCT02180295 (first received 2 July 2014).
NCT02526745 {unpublished data only}
- NCT02526745. Safety and immunogenicity study of live attenuated vaccine against herpes zoster in Chinese adults aged 50 years and older. clinicaltrials.gov/ct2/show/NCT02526745 (first received 18 August 2015).
NCT03116594 {unpublished data only}
- NCT03116594. Immunogenicity and safety of two lots of NBP608 compared to Zostavax in healthy adult aged 50 and over. clinicaltrials.gov/ct2/show/NCT03116594 (first received 17 April 2017).
NCT04091451 {unpublished data only}
- NCT04091451. A safety and immunogenicity study of GSK Biologicals' herpes zoster subunit vaccine (HZ/su) GSK1437173A on a two-dose schedule in adults ≥ 50 years of age with a prior episode of herpes zoster. clinicaltrials.gov (first received 19 April 2017).
NCT04210752 {unpublished data only}
- NCT04210752. A phase 1, randomised, activator-controlled, double-blind, parallel study to assess the safety, tolerability and explore the immunogenicity of EG-HZ in healthy adult volunteers. clinicaltrials.gov/ct2/show/NCT04210752 (first received 24 February 2020).
NCT04869982 {unpublished data only}
- NCT04869982. A phase IV, randomized, observer-blind, placebo-controlled, multi-center study to assess the prophylactic efficacy against herpes zoster, immunogenicity and safety of Shingrix when administered intramuscularly on a 2-dose schedule in Chinese adults aged 50 years and older. clinicaltrials.gov/study/NCT04869982 (first received 3 May 2021).
NCT05007041 {unpublished data only}
- NCT05007041. Safety of simultaneous vaccination with zoster vaccine recombinant (RZV) and quadrivalent adjuvanted inactivated influenza vaccine (aIIV4). clinicaltrials.gov/ct2/show/NCT05007041 (first received 21 September 2021).
NCT05047770 {unpublished data only}
- NCT05047770. A phase III, randomized, open-label, controlled, multicenter study to evaluate the immune response and safety of both herpes zoster subunit vaccine in healthy adults aged 50 years and older AND the influenza virus vaccine in healthy adults aged 18 years and older when administered sequentially or coadministered with mRNA-1273 booster vaccination. clinicaltrials.gov/ct2/show/NCT05047770 (first received 17 September 2021).
NCT05219253 {unpublished data only}
- NCT05219253. A phase 3, randomised, observer-blind, placebo-controlled, multi-centre study to evaluate the immune response and safety of the herpes zoster subunit vaccine when administered intramuscularly on a 2-dose schedule in adults aged 50 years and older in India. clinicaltrials.gov/ct2/show/NCT05219253 (first received 2 February 2018).
NCT05245838 {unpublished data only}
- NCT05245838. A phase 1 randomized, subject-blinded, active-controlled, dose escalation, multicenter trial to evaluate the safety, tolerability, and immunogenicity of an investigational herpes zoster vaccine (Z-1018) compared to Shingrix® in healthy adult volunteers between the ages of 50 and 69 years. clinicaltrials.gov/ct2/show/NCT05245838 (first received 18 February 2022).
Additional references
Agmon‐Levin 2009
Amanna 2007
Aps 2018
Arvin 2005
- Arvin A. Ageing, immunity, and the varicella-zoster virus. New England Journal of Medicine 2005;352(22):2266-7. [PMID: ] - PubMed
Asherson 2006
- Asherson RA. The catastrophic antiphospholipid (Asherson's) syndrome. Autoimmunity Reviews 2006;6(2):64-7. [MEDLINE: ] - PubMed
Atkins 2004
Baldridge 2004
- Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D, el al. Taking a toll on human disease: toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opinion on Biological Therapy 2004;4(7):1129-38. [PMID: ] - PubMed
Balofsky 2010
Blom 2019
- Blom K, Yin L, Arnheim-Dahlström L. Effectiveness of the herpes zoster vaccine Zostavax® in Stockholm County, Sweden. Vaccine 2019;37(31):4401-6. [DOI: ] - PubMed
Bruijn 2007
Caputo 2019
- Caputo M, Horn J, Karch A, Akmatov MK, Becher H, Braun B, et al. Herpes zoster incidence in Germany - an indirect validation study for self-reported disease data from pretest studies of the population-based German National Cohort. BMC Infectious Diseases 2019;19(1):99. [DOI: 10.1186/s12879-019-3691-2] - DOI - PMC - PubMed
Cho 2007
- Cho JW, Shin DH, Lee KS. Polymorphism of the IL-10 gene is associated with susceptibility to herpes zoster in Korea. Journal of Dermatological Science 2007;45(3):213-5. [PMID: ] - PubMed
Chung 2016
Coplan 2004
- Coplan PM, Schmader K, Nikas A, Chan ISF, Choo P, Levin MJ, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the Brief Pain Inventory. Journal of Pain 2004;5(6):344-56. [DOI: 10.1016/j.jpain.2004.06.001] - DOI - PubMed
Dagnew 2021
- Dagnew AF, Klein NP, Hervé C, Kalema G, Di Paolo E, Peterson J, et al. The adjuvanted recombinant zoster vaccine in adults aged ≥65 years previously vaccinated with a live attenuated herpes zoster vaccine. Journal of Infectious Diseases 2021;224:1139-46. [DOI: DOI: 10.1093/infdis/jiaa083] - PMC - PubMed
Dworkin 2003
FDA 2006
- USA FDA approval letter - Zostavax, 25 May 2006. https://wayback.archive-it.org/7993/20170723093336/https://www.fda.gov/B... accessed 24 October 2019.
FDA 2017
- USA FDA approval letter - zoster vaccine recombinant, adjuvanted. 20 October 2017. www.fda.gov/downloads/biologicsblood vaccines/vaccines/approvedproducts/ucm581750.pdf accessed 24 October 2019.
Garçon 2007
- Garçon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Review of Vaccines 2007;6(5):723-39. [MEDLINE: ] - PubMed
Gatti 2010
- Gatti A, Pica F, Boccia MT, De Antoni F, Sabato AF, Volpi A. No evidence of family history as a risk factor for herpes zoster in patients with post-herpetic neuralgia. Journal of Medical Virology 2010;82(6):1007-11. [PMID: ] - PubMed
Gilden 2000
- Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ. Neurologic complications of the reactivation of varicella zoster virus. New England Journal of Medicine 2000;342(9):635–45. [PMID: ] - PubMed
Glenny 2005
- Glenny AM, Altman DG, Sakarovitch C, Deeks JJ, D'Amico R, Bradburn M, et al. Indirect comparisons of competing interventions. Health Technology Assessment 2005;9(26):1-134. [PMID: ] - PubMed
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 1 December 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Gruver 2007
Guyatt 2006a
- Guyatt G, Gutterman D, Baumann MH, Addrizzo-Harris D, Hylek EM, Phillips B, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians Task Force. Chest 2006;129(1):174-81. [PMID: ] - PubMed
Guyatt 2006b
- Guyatt G, Vist G, Falck-Ytter Y, Kunz R, Magrini N, Schünemann H. An emerging consensus on grading recommendations? ACP Journal Club 2006;144(1):A8-9. [PMID: ] - PubMed
Haanpää 2002
- Haanpää M, Nurmikko T, Hurme M. Polymorphism of the IL-10 gene is associated with susceptibility to herpes zoster. Scandinavian Journal of Infectious Diseases 2002;34(2):112-4. [PMID: ] - PubMed
Hawkes 2015
Heymann 2008
- Heymann AD, Chodick G, Karpati T, Kamer L, Kremer E, Green MS, et al. Diabetes as a risk factor for herpes zoster infection: results of a population-based study in Israel. Infection 2008;36(3):226-30. [PMID: ] - PubMed
Hicks 2008
- Hicks LD, Cook-Norris RH, Mendoza N, Madkan V, Arora A, Tyring SK. Family history as a risk factor for herpes zoster: a case-control study. Archives of Dermatology 2008;144(5):603-8. [PMID: ] - PubMed
Higgins 2003
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Ikematsu 2018
- Ikematsu H, Yamashita N, Ogawa M, Hirano M, Kovac M, Watanabe D. Efficacy, safety and immunogenicity of new adjuvanted herpes zoster subunit vaccine for Japanese over 50 years old and over 70 years old. Kansenshogaku Zasshi 2018;92(2):103-14.
Jansen 2013
Jih 2009
- Jih JS, Chen YJ, Lin MW, Chen YC, Chen TJ, Huang YL, et al. Epidemiological features and costs of herpes zoster in Taiwan: a national study 2000 to 2006. Acta Dermato-Venereologica 2009;89(6):612-6. [PMID: ] - PubMed
Johnson 2014
Johnson 2020
- Johnson RW, Levin MJ. Vaccines for older adults: current practices and future opportunities. In: Weinberger B, editors(s). Interdisciplinary Topics in Gerontology and Geriatrics. Herpes Zoster and Its Prevention by Vaccination edition. Vol. 43. Basel: Current Vaccines for the Older Population, 2020:131–45. [DOI: ] - PubMed
Kawai 2014
Kawai 2016
Kicinski 2013
Langan 2013
Latour 1997
- Latour J, Abraira V, Cabello JB, López Sánchez J. Investigation methods in clinical cardiology (IV) clinical measurements in cardiology: validity and errors of measurement. Revista Española de Cardiología 1997;50(2):117-28. [PMID: ] - PubMed
Lay 2015
- Lai YC, Yew YW. Severe autoimmune adverse events post herpes zoster vaccine: a case-control study of adverse events in a national database. Journal of Drugs in Dermatology 2015;14(7):681-4. [PMID: ] - PubMed
Le Dantec 2015
- Le Dantec C, Brooks WH, Renaudineau Y. Epigenomic revolution in autoimmune diseases. World Journal of Immunology 2015;5(2):62-7. [DOI: 10.5411/wji.v5.i2.62] - DOI
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Leung 2022
- Leung J, Dooling K, Marin M, Anderson TC, Harpaz R. The impact of universal varicella vaccination on herpes zoster incidence in the United States: comparison of birth cohorts preceding and following varicella vaccination program launch. Journal of Infectious Diseases 2022;226(Suppl 4):470–7. [DOI: ] - PubMed
Lewis 1993
Mareque 2019
Marra 2016
Marra 2022
McGirr 2019
- McGirr A, Widenmaier R, Curran D, Espié E, Mrkvan T, Oostvogels L, et al. The comparative efficacy and safety of herpes zoster vaccines: a network meta-analysis. Vaccine 2019;37:2896-909. [DOI: ] - PubMed
Moffat 2007
- Moffat J, Ku CC, Zerboni L, Sommer M, Arvin A. Pathogenesis and the disease consequences of primary infection. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, et al, editors(s). Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press, 2007. [PMID: ] - PubMed
NBP608
- SK Chemicals receives permission to market shingles vaccine. http://english.hani.co.kr/arti/english_edition/e_business/813943.html accessed 24 October 2019.
Newell 1992
Partridge 2009
- Partridge DG, McKendrick MW. The treatment of varicella-zoster virus infection and its complications. Expert Opinion on Pharmacotherapy 2009;10(5):797-812. [PMID: ] - PubMed
Patil 2022
Perricone 2013
- Perricone C, Colafrancesco S, Mazor RD, Soriano A, Agmon-Levin N, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) 2013: unveiling the pathogenic, clinical and diagnostic aspects. Journal of Autoimmunity 2013;47(December):1-16. [DOI: 10.1016/j.jaut.2013.10.004] - DOI - PubMed
Pickering 2011
Rajesh 1995
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rimland 2010
- Rimland D, Moanna A. Increasing incidence of herpes zoster among veterans. Clinical Infectious Diseases 2010;50(7):1000-5. [PMID: ] - PubMed
Sampathkumar 2009
Sanford 2010
- Sanford M, Keating GM. Zoster vaccine (Zostavax®) a review of its use in preventing herpes zoster and postherpetic neuralgia in older adults. Drugs and Aging 2010;27(2):159-76. [1170-229X/10/0002-0159/$49.95/0] - PubMed
Schmader 2007
- Schmader KE, Sloane R, Pieper C, Coplan PM, Nikas A, Saddier P, et al. Herpes zoster pain and discomfort on functional status and quality of life in older adults. Clinical Journal of Pain 2007;23(6):490-6. [PMID: ] - PubMed
Schmader 2012
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Sengupta 2008
- Sengupta N, Booy R, Schmitt HJ, Peltola H, van-Damme P, Schumacher RF, et al. Varicella vaccination in Europe: are we ready for a universal childhood programme? European Journal of Pediatrics 2008;167:47–55. [DOI: ] - PubMed
Sun 2011
- Sun W. Summary basis for regulatory action. http://wayback.archive-it.org/7993/20170723093318/https://www.fda.gov/do... 2011.
Thomas 2004
- Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infectious Diseases 2004;4(1):26-33. [PMID: ] - PubMed
Tricco 2018
Watad 2018
- Watad A, Quaresma M, Bragazzi NL, Cervera R, Tervaert JW, Amital H, et al. The autoimmune/inflammatory syndrome induced by adjuvants (ASIA)/Shoenfeld's syndrome: descriptive analysis of 300 patients from the international ASIA syndrome registry. Clinical Rheumatology 2018;37(2):483-93. [DOI: 10.1007/s10067-017-3748-9] - DOI - PubMed
WHO 2014
- Varicella and herpes zoster vaccines: WHO position paper, June 2014. C:/Users/acer/Downloads/WER8925_265-287WHO.pdf:265-87.
Wise 2000
- Wise RP, Salive ME, Braun MM, Mootrey GT, Seward JF, Rider LG, et al. Postlicensure safety surveillance for varicella vaccine. JAMA 2000;284(10):1271-9. [MEDLINE: ] - PubMed
References to other published versions of this review
Gagliardi 2010
Gagliardi 2012
Gagliardi 2016
Gagliardi 2019
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous