Cerebrolysin for acute ischaemic stroke - PubMed (original) (raw)
Cerebrolysin for acute ischaemic stroke
Liliya Eugenevna Ziganshina et al. Cochrane Database Syst Rev. 2023.
Abstract
Background: Cerebrolysin is a mixture of low-molecular-weight peptides and amino acids derived from porcine brain, which has potential neuroprotective properties. It is widely used in the treatment of acute ischaemic stroke in Russia, Eastern Europe, China, and other Asian and post-Soviet countries. This is an update of a review first published in 2010 and last updated in 2020.
Objectives: To assess the benefits and harms of Cerebrolysin or Cerebrolysin-like agents for treating acute ischaemic stroke.
Search methods: We searched the Cochrane Stroke Trials Register, CENTRAL, MEDLINE, Embase, Web of Science Core Collection, with Science Citation Index, and LILACS in May 2022 and a number of Russian databases in June 2022. We also searched reference lists, ongoing trials registers, and conference proceedings.
Selection criteria: Randomised controlled trials (RCTs) comparing Cerebrolysin or Cerebrolysin-like agents started within 48 hours of stroke onset and continued for any length of time, with placebo or no treatment in people with acute ischaemic stroke.
Data collection and analysis: Three review authors independently applied the inclusion criteria, assessed trial quality and risk of bias, extracted data, and applied GRADE criteria to the evidence.
Main results: Seven RCTs (1773 participants) met the inclusion criteria of the review. In this update we added one RCT of Cerebrolysin-like agent Cortexin, which contributed 272 participants. We used the same approach for risk of bias assessment that was re-evaluated for the previous update: we added consideration of the public availability of study protocols and reported outcomes to the selective outcome reporting judgement, through identification, examination, and evaluation of study protocols. For the Cerebrolysin studies, we judged the risk of bias for selective outcome reporting to be unclear across all studies; for blinding of participants and personnel to be low in three studies and unclear in the remaining four; and for blinding of outcome assessors to be low in three studies and unclear in four studies. We judged the risk of bias for generation of allocation sequence to be low in one study and unclear in the remaining six studies; for allocation concealment to be low in one study and unclear in six studies; and for incomplete outcome data to be low in three studies and high in the remaining four studies. The manufacturer of Cerebrolysin supported three multicentre studies, either totally, or by providing Cerebrolysin and placebo, randomisation codes, research grants, or statisticians. We judged two studies to be at high risk of other bias and the remaining five studies to be at unclear risk of other bias. We judged the study of Cortexin to be at low risk of bias for incomplete outcome data and at unclear risk of bias for all other domains. All-cause death: Cerebrolysin or Cortexin probably result in little to no difference in all-cause death (risk ratio (RR) 0.96, 95% confidence interval (CI) 0.65 to 1.41; 6 trials, 1689 participants; moderate-certainty evidence). None of the included studies reported on poor functional outcome, defined as death or dependence at the end of the follow-up period, early death (within two weeks of stroke onset), quality of life, or time to restoration of capacity for work. Only one study clearly reported on the cause of death: cerebral infarct (four in the Cerebrolysin and two in the placebo group), heart failure (two in the Cerebrolysin and one in the placebo group), pulmonary embolism (two in the placebo group), and pneumonia (one in the placebo group). Non-death attrition (secondary outcome): Cerebrolysin or similar peptide mixtures may result in little to no difference in non-death attrition, but the evidence is very uncertain, with a considerable level of heterogeneity (RR 0.72, 95% CI 0.38 to 1.39; 6 trials, 1689 participants; very low-certainty evidence). Serious adverse events (SAEs): Cerebrolysin probably results in little to no difference in the total number of people with SAEs (RR 1.16, 95% CI 0.81 to 1.66; 3 trials, 1335 participants; moderate-certainty evidence). This comprised fatal SAEs (RR 0.90, 95% CI 0.59 to 1.38; 3 trials, 1335 participants; moderate-certainty evidence) and an increase in the total number of people with non-fatal SAEs (RR 2.39, 95% CI 1.10 to 5.23; 3 trials, 1335 participants; moderate-certainty evidence). In the subgroup of dosing schedule 30 mL for 10 days (cumulative dose 300 mL), the increase was more prominent (RR 2.87, 95% CI 1.24 to 6.69; 2 trials, 1189 participants). Total number of people with adverse events: Cerebrolysin or similar peptide mixtures may result in little to no difference in the total number of people with adverse events (RR 1.03, 95% CI 0.92 to 1.14; 4 trials, 1607 participants; low-certainty evidence).
Authors' conclusions: Moderate-certainty evidence indicates that Cerebrolysin or Cerebrolysin-like peptide mixtures derived from cattle brain probably have no beneficial effect on preventing all-cause death in acute ischaemic stroke. Moderate-certainty evidence suggests that Cerebrolysin probably has no beneficial effect on the total number of people with serious adverse events. Moderate-certainty evidence also indicates a potential increase in non-fatal serious adverse events with Cerebrolysin use.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
LEZ: is a Cochrane director (Cochrane Russia). She was not involved in the editorial process.
TRA: none known.
DN: none known.
KI: none known.
Figures
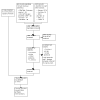
1
Study flow diagram
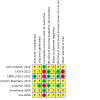
2
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
1.1. Analysis
Comparison 1: Cerebrolysin or Cortexin versus placebo, Outcome 1: All‐cause death
1.2. Analysis
Comparison 1: Cerebrolysin or Cortexin versus placebo, Outcome 2: Non‐death attrition
1.3. Analysis
Comparison 1: Cerebrolysin or Cortexin versus placebo, Outcome 3: Total number of people with SAEs
1.4. Analysis
Comparison 1: Cerebrolysin or Cortexin versus placebo, Outcome 4: Total number of people with fatal SAEs
1.5. Analysis
Comparison 1: Cerebrolysin or Cortexin versus placebo, Outcome 5: Total number of people with non‐fatal SAEs
1.6. Analysis
Comparison 1: Cerebrolysin or Cortexin versus placebo, Outcome 6: Total number of people with adverse events
2.1. Analysis
Comparison 2: Sensitivity analyses: Cerebrolysin or Cortexin versus placebo, Outcome 1: All‐cause death. Sensitivity 1. Best‐case
2.2. Analysis
Comparison 2: Sensitivity analyses: Cerebrolysin or Cortexin versus placebo, Outcome 2: All‐cause death. Sensitivity 2. Worst‐case
2.3. Analysis
Comparison 2: Sensitivity analyses: Cerebrolysin or Cortexin versus placebo, Outcome 3: All‐cause death. Sensitivity 3. Complete case (missing data excluded)
2.4. Analysis
Comparison 2: Sensitivity analyses: Cerebrolysin or Cortexin versus placebo, Outcome 4: All‐cause death. Sensitivity 4. Risk of bias
Update of
- Cerebrolysin for acute ischaemic stroke.
Ziganshina LE, Abakumova T, Hoyle CH. Ziganshina LE, et al. Cochrane Database Syst Rev. 2020 Jul 14;7(7):CD007026. doi: 10.1002/14651858.CD007026.pub6. Cochrane Database Syst Rev. 2020. PMID: 32662068 Free PMC article. Updated.
Similar articles
- Cerebrolysin for acute ischaemic stroke.
Ziganshina LE, Abakumova T, Vernay L. Ziganshina LE, et al. Cochrane Database Syst Rev. 2017 Apr 21;4(4):CD007026. doi: 10.1002/14651858.CD007026.pub5. Cochrane Database Syst Rev. 2017. PMID: 28430363 Free PMC article. Updated. - Cerebrolysin for acute ischaemic stroke.
Ziganshina LE, Abakumova T, Vernay L. Ziganshina LE, et al. Cochrane Database Syst Rev. 2016 Dec 5;12(12):CD007026. doi: 10.1002/14651858.CD007026.pub4. Cochrane Database Syst Rev. 2016. PMID: 27918088 Free PMC article. Updated. - Cerebrolysin for acute ischaemic stroke.
Ziganshina LE, Abakumova T, Hoyle CH. Ziganshina LE, et al. Cochrane Database Syst Rev. 2020 Jul 14;7(7):CD007026. doi: 10.1002/14651858.CD007026.pub6. Cochrane Database Syst Rev. 2020. PMID: 32662068 Free PMC article. Updated. - Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.
Weibel S, Rücker G, Eberhart LH, Pace NL, Hartl HM, Jordan OL, Mayer D, Riemer M, Schaefer MS, Raj D, Backhaus I, Helf A, Schlesinger T, Kienbaum P, Kranke P. Weibel S, et al. Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article. - Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.
Sbidian E, Chaimani A, Garcia-Doval I, Do G, Hua C, Mazaud C, Droitcourt C, Hughes C, Ingram JR, Naldi L, Chosidow O, Le Cleach L. Sbidian E, et al. Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. PMID: 29271481 Free PMC article. Updated.
Cited by
- Conservative and newer drug treatment for degenerative cervical myelopathy.
Ede O, Cheung JPY. Ede O, et al. J Clin Orthop Trauma. 2025 Mar 14;64:102972. doi: 10.1016/j.jcot.2025.102972. eCollection 2025 May. J Clin Orthop Trauma. 2025. PMID: 40191170 - Life-Threatening Anaphylaxis due to Cerebrolysin®.
Trimmel H, Tauber W, Zikeli M. Trimmel H, et al. Case Rep Neurol Med. 2024 Jul 18;2024:2332908. doi: 10.1155/2024/2332908. eCollection 2024. Case Rep Neurol Med. 2024. PMID: 39055722 Free PMC article. - Heterogeneous treatment effects of Cerebrolysin as an early add-on to reperfusion therapy: post hoc analysis of the CEREHETIS trial.
Kalinin MN, Khasanova DR. Kalinin MN, et al. Front Pharmacol. 2024 Jan 5;14:1288718. doi: 10.3389/fphar.2023.1288718. eCollection 2023. Front Pharmacol. 2024. PMID: 38249342 Free PMC article. - Synergistic effects of neuroprotective drugs with intravenous recombinant tissue plasminogen activator in acute ischemic stroke: A Bayesian network meta-analysis.
Dang C, Wang Q, Zhuang Y, Li Q, Lu Y, Xiong Y, Feng L. Dang C, et al. PLoS One. 2024 Dec 2;19(12):e0311231. doi: 10.1371/journal.pone.0311231. eCollection 2024. PLoS One. 2024. PMID: 39621713 Free PMC article.
References
References to studies included in this review
Amiri Nikpour 2014 {published data only}
- Nazarbaghi S, Amiri Nikpoor M. Acute stroke management efficiency of cerebrolysin in treatment of patients with acute ischemic stroke. International Journal of Stroke 2014;9 Suppl 3:91-2.
CASTA 2012 {published data only}
- Bornstein NM. CASTA - the background for the study and protocol design. International Journal of Stroke 2010;5 Suppl 2:50.
- Brainin M, Heiss WD, Bornstein N, Tuomilehto J, Hong Z. CASTA - results of a double-blind, placebo-controlled, randomized trial with cerebrolysin on patients with acute ischaemic stroke in Asia. Cerebrovascular Diseases 2012;33 Suppl 2:459-60.
- Heiss WD, Brainin M, Bornstein N, Zhen H. Cerebrolysin in patients with acute ischemic stroke - the CASTA study. International Journal of Stroke 2010;5 Suppl 2:71.
- Heiss WD, Brainin M, Bornstein NM, Tuomilehto J, Hong Z, Cerebrolysin Acute Stroke Treatment in Asia (CASTA) Investigators. Cerebrolysin in patients with acute ischemic stroke in Asia: results of a double-blind, placebo-controlled randomized trial. Stroke 2012;43(3):630-6. - PubMed
- Hong Z, Moessler H, Bornstein N, Brainin M, Heiss W-D, CASTA Investigators. A double-blind, placebo-controlled, randomized trial to evaluate the safety and efficacy of cerebrolysin in patients with acute ischaemic stroke in Asia - CASTA. International Journal of Stroke 2009;4(5):406-12. - PubMed
CERE‐LYSE‐1 2012 {published data only}
- Lang W, Stadler CH, Poljakovic Z, Fleet D, Lyse Study Group. A prospective, randomized, placebo-controlled, double-blind trial about safety and efficacy combined treatment with alteplase (rt-PA) and cerebrolysin in acute ischaemic hemispheric stroke. International Journal of Stroke 2012;8(2):95-104. - PubMed
- NCT00840671. Combined treatment with alteplase (Rt-PA) and cerebrolysin® in acute ischemic hemispheric stroke (CERE-LYSE). clinicaltrials.gov/show/NCT00840671 (first received 10 February 2009).
Cortexin‐Shamalov 2014 {published data only}17707356https://www.elibrary.ru/item.asp?id=17707356
- Aliferova VM, Dadasheva MN, Doronin BM, Kovalenko AV, Lokshtanova TM, Martynov MYu, et al. Clinical efficacy and pharmacoeconomic characteristics of the neuroprotection with low doses of cortexin in the treatment of acute ischemic stroke [Клиническая эффективность и фармакоэкономические характеристики нейропротекции низкими дозами кортексина в терапии острого ишемического инсульта]. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2014;4:41‑6. [ELIBRARY ID: 21538285] [ELIBRARY.RU: https://elibrary.ru/item.asp?id=21538285] - PubMed
- Stakhovskaya LV, Meshkova KS, Dadasheva MN, Chefranova JYu, Titova LP, Lokshtanova TM, et al. Results of multicenter randomize prospective double-blind placebo-controlled study of the safety and efficacy of cortexin in acute and subacute periods of hemispheric ischemic stroke [Многоцентровое рандомизированное проспективное двойное слепое плацебо контролируемое исследование безопасности и эффективности кортексина в остром и раннем восстановительном периоде полушарного ишемического инсульта]. Vestnik Rossiiskoy Voenno-Meditsinskoy Academii [Bulletin of the Russian Military Medical Academy] 2012;1(37):238-44. [ELIBRARY ID: 17707356] [ELIBRARY.RU: https://www.elibrary.ru/item.asp?id=17707356]
Ladurner 2005 {published data only}
- Ladurner G, Gmeinbauer R, Moessler H. Cerebrolysin in acute ischaemic stroke: a randomized, placebo-controlled trial with a neuroprotective agent. Cerebrovascular Diseases 2001;11(4):75.
- Ladurner G, Kalvach P, Moessler H, Cerebrolysin Study Group. Neuroprotective treatment with cerebrolysin in patients with acute stroke: a randomised controlled trial. Journal of Neural Transmission 2005;112(3):415-28. - PubMed
- Ladurner G. Neuroprotection in acute ischaemic stroke. Stroke 2001;32 Suppl 1:323.
Skvortsova 2004 {published data only}
- Skvortsova VI, Shamalov NA, Stakhovskaya LV, Gubsky LV, Tikhonova IV, Smichkov AS. Cerebrolysin in acute ischaemic stroke: results of randomised, double blind, placebo-controlled study. Cerebrovascular Diseases 2005;19 Suppl 2:76.
- Skvortsova VI, Stakhovskaia LV, Gubskii LV, Shamalov NA, Tikhonova IV, Smychkov AS. A randomized, double-blind, placebo-controlled study of Cerebrolysin safety and efficacy in the treatment of acute ischemic stroke. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2004;Suppl 11:51-5. - PubMed
- Skvortsova VI, Stakhovskaya LV, Gubsky LV, Shamalov NA, Tikhonova IV, Smychkov AS. Evaluation of safety and efficacy of cerebrolysin for treating acute ischaemic stroke [Issledovaniye bezopasnosti i effektivnosti tserebrolizina dlya lecheniya ostrogo ishemicheskogo insul’ta]. Рецепт [Prescription] 2007;5(55):94-8.
Xue 2016 {published data only}
- NCT02149875. Dl-3-n-butylphthalide and cerebrolysin treatment in acute ischemic stroke. clinicaltrials.gov/show/NCT02149875 (first received 29 May 2014).
References to studies excluded from this review
Belova 2018 {published data only}
- Belova LA, Mashin VV, Abramova VV, Slastyon EYu, Belov DV. Efficacy of cortexin in the acute period of hemispheric ischemic stroke [Эффективность кортексина в остром периоде полушарного ишемического инсульта]. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2018;7:30-4. [DOI: ] - PubMed
Bogolepova 2019 {published data only}
- Bogolepova AN. Possibilities of neurotrophic therapy in early recovery after stroke [Возможности нейротрофической терапии в раннем восстановлении после инсульта]. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2019;119(8):84-9. [DOI: ] - PubMed
CEREC‐Stroke {published data only}
- ISRCTN15233803. Evaluation of efficacy and safety of Cerebrolysin as add-on therapy in patients with acute stroke after failed recanalisation therapy. https://www.isrctn.com/ISRCTN15233803 (first received 25 May 2020). [DOI: ]
Gharagozli 2017 {published data only}
- Gharagozli K, Harandi AA, Houshmand S, Akbari N, Muresanu DF, Vester J, et al. Efficacy and safety of Cerebrolysin treatment in early recovery after acute ischemic stroke: a randomized, placebo-controlled, double-blinded, multicenter clinical trial. Journal of Medicine and Life 2017;10(3):153-60. - PMC - PubMed
- IRCT138803272042N1. The efficacy of cerebrolysin in the treatment of acute ischemic stroke. en.irct.ir/trial/1663 (first received 29 August 2009).
Kurenkova 2014 {published data only}
- Kurenkova NA, Filatova MS, Popov DV, Makarova LD, Makhankov OV. Efficiency of Cortexin in early rehabilitation of patients with ischemic stroke [Эффективность Кортексина при ранней реабилитации больных с ишемическим инсультом]. Russkii Meditsinskii Zhurnal [Russian Medical Journal] 2014 ;10:748. [RMJ: https://www.rmj.ru/articles/klinicheskaya_farmakologiya/Effektivnosty_Ko...]
NCT04904341 {published data only}
- NCT04904341. Efficacy of Cerebrolysin treatment as an add-on therapy to mechanical thrombectomy in acute ischemic stroke. https://classic.clinicaltrials.gov/ct2/show/NCT04904341 (first received 27 May 2021). [CLINICALTRIALS.GOV IDENTIFIER: NCT04904341]
Slyusar 2021 {published data only}
- Slyusar O, Maximov I, Babaskina L, Lobuteva L. Ineffective drugs: Cerebrolysin and piracetam. Research Journal of Pharmacy and Technology 2021;14:2643-8. [DOI: 10.52711/0974-360X.2021.00466] - DOI
References to ongoing studies
CEREHETIS {published data only}
- ISRCTN87656744. Cerebrolysin to prevent intracranial bleeding after intravenous thrombolysis (blood clot) in patients with ischemic stroke. https://www.isrctn.com/ISRCTN87656744 (first received 16 February 2021). [DOI: ]
- Kalinin M, Khasanova D. Cerebrolysin as an early add-on to reperfusion therapy: risk of hemorrhagic transformation after ischemic stroke (CEREHETIS). A prospective, randomized, active control, multicenter pilot study. European Stroke Journal 2021;6(1 Suppl):35. [DOI: 10.1177/23969873211034932] - DOI - PMC - PubMed
CERE‐REHA‐RU/01 {published data only}
- CERE-REHA-RU/01. Double-blind placebo-controlled multi-centre randomised parallel group clinical study of phase IV on effectiveness of adding cerebrolysin to standard rehabilitation complex interventions in patients with ischaemic stroke. grls.rosminzdrav.ru/CIPermissionMini.aspx?CIStatementGUID=70440249-c5c0-... (first received 19 January 2015).
GP20011‐P4‐32 {published data only}
- GP20011-P4-32. Multicenter, double-blind, placebo-controlled, randomized, in two parallel groups study of the efficacy of Cortexin®, 10 mg, manufactured by GEROPHARM LLC, Russia, in patients in the acute period of ischemic stroke [Многоцентровое двойное слепое плацебо контролируемое рандомизированное в двух параллельных группах исследование эффективности лекарственного препарата Кортексин®, 10 мг, производства ООО «ГЕРОФАРМ», Россия, у пациентов в остром периоде ишемического инсульта]. https://grls.rosminzdrav.ru/CIPermissionMini.aspx?CIStatementGUID=71c142... (first received 29 August 2019).
GP20011‐P4‐36 {published data only}
- GP20011-P4-36. Multicenter, double-blind, randomized, controlled, parallel study of the therapeutic equivalence of intramuscular and intravenous forms of administration of the drug Cortexin®, 10 mg, produced by GEROPHARM LLC, Russia, in relation to the degree of functional recovery in patients in the acute period of ischemic stroke [Многоцентровое двойное слепое рандомизированное контролируемое параллельное исследование терапевтической эквивалентности внутримышечной и внутривенной форм введения лекарственного препарата Кортексин®, 10 мг, производства ООО «ГЕРОФАРМ», Россия, в отношении степени функционального восстановления у пациентов в остром периоде ишемического инсульта]. https://grls.rosminzdrav.ru/CIPermissionMini.aspx?CIStatementGUID=5ee044... (first received 2 December 2021).
IRCT201406169014N36 {published and unpublished data}
- IRCT201406169014N36. The effect of cerebrolysin versus placebo on improvement of patients with acute ischemic stroke: a double blinded randomized clinical trial. en.irct.ir/trial/9475 (first received 23 March 2014).
ISRCTN54581790 (ESCAS) {published data only}
- ISRCTN54581790. Efficacy and safety of Cerebrolysin in the treatment of aphasia after acute ischemic stroke (ESCAS). https://www.isrctn.com/ISRCTN54581790 (first received 29 April 2020). [DOI: ]
ISRCTN88122184 (CODEC) {published data only}
- ISRCTN88122184. Can treatment with Cerebrolysin improve recovery after acute ischemic stroke? https://www.isrctn.com/ISRCTN88122184 (first received 29 April 2020). [DOI: ]
NCT05124353 {published data only}
- NCT05124353. Evaluation of the effect of early administration of neuroprotective drug (Cerebrolysin) on the outcome of patients with acute ischemic stroke undergoing endovascular therapy. https://clinicaltrials.gov/ct2/show/record/NCT05124353 (first received 17 November 2021).
Additional references
AHA 2019
AHA 2022
Alper 2015
Alvarez 2000
- Alvarez XA, Lombardi VR, Corzo L, Pérez P, Pichel V, Laredo M, et al. Oral Cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. Journal of Neural Transmission 2000;59:315-28. - PubMed
Appelros 2015
Bajenaru 2010
Bath 2004a
Bath 2004b
Bath 2004c
Bath 2013
Bath 2014
Bath 2017
Bereczki 2007
Bereczki 2008
Bereczki 2017
Berge 2002
Boncoraglio 2019
Bornstein 2018
Brunström 2015
Candelise 2001
Chang 2014
Chang 2016
Chen 2008
Chukanova 2005
- Chukanova EI. The effect of Cerebrolysin on the clinical symptoms and the course of ischemic encephalopathy. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2005;105(1):42-5. - PubMed
Ciccone 2014
Covidence
- Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org (accessed May 2023).
Cui 2019
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Emberson 2014
- Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014;384(9958):1929-35. - PMC - PubMed
European Ad Hoc Consensus 1998
- The European Ad Hoc Consensus Group. Neuroprotection as initial therapy in acute stroke. Third report of an ad hoc consensus group meeting. Cerebrovascular Diseases 1998;8(1):59-72. - PubMed
Feng 2011
Fragoso 2002
- Fragoso Y, Dantas DC. Cerebrolysin for Alzheimer's disease. Cochrane Database of Systematic Reviews 2002, Issue 3. Art. No: CD003801. [DOI: 10.1002/14651858.CD003801] - DOI
French 2007
French 2016
Gafurov 2004
- Gafurov BG, Alikulova NA. Clinical and pathogenetical peculiarities and treatment policy in ischemic stroke of elderly and old age. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2004;104 Suppl 11:44-6. - PubMed
Gandolfo 2002
GBD Stroke Collaborators 2019
Geeganage 2010
Gevaert 2015
- Gevaert B, D'Hondt M, Bracke N, Yao H, Wyendaele E, Vissers JPC, et al. Peptide profiling of Internet-obtained Cerebrolysin using high performance liquid chromatography – electrospray ionization ion trap and ultra high performance liquid chromatography – ion mobility – quadrupole time of flight mass spectrometry. Drug Testing and Analysis 2015;7:835-42. [DOI: 10.1002/dta.1817] - DOI - PubMed
Ginsberg 2016
Goenka 2019
Gomazkov 2015
- Gomazkov OA. Cortexin: molecular mechanisms and targets of neuroprotective activity [Кортексин: молекулярные механизмы и мишени нейропротективнойактивности]. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2015;8:99-104. [DOI: 10.17116/jnevro20151158199-104] - DOI - PubMed
GovRu 2019
- Government of the Russian Federation. Essential Medicines List for 2019 [Правительство российской федерации]. static.government.ru/media/files/K1fPEUszF2gmvwTkw74iPOASarj7KggI.pdf (accessed prior to 23 June 2020).
GovRu 2022
- Government of the Russian Federation. Corrections to Essential Medicines List for 2019 [Правительство Российской Федерации]. static.government.ru/media/files/gGR64GGr0trcTnxAVXA0y4KwW9iUHGtI.pdf 23 December 2021 (accessed May 2023).
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed prior to 27 June 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Gromova 2006
- Gromova OA, Tret'iakov VE, Moshkovskiĭ SA, Gusev EI, Nikonov AA, Val'kova LA, et al. An oligopeptide membrane fraction of Cerebrolysin. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2006;106(7):68-70. - PubMed
Guekht 2015
- Guekht A, Heiss D, Gusev E, Vester J, Doppler E, Muresanu D. Cerebrolysin and recovery after stroke (CARS 2): a randomized, placebo-controlled, double-blind, multicenter clinical study. Journal of the Neurological Sciences 2015;357:e103. [DOI: ]
Gulyaeva 2019
- Gulyaeva NV. Molecular mechanisms of the actions of brain peptide-containing drugs: Cortexin. Neuroscience and Behavioral Physiology 2019;49:1067-70. [DOI: 10.1007/s11055-019-00839-4] - DOI
Gusev 2003
- Gusev EI, Skvortsova VI, Stakhovskaia LV. Epidemiology of stroke in Russia. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2003;Suppl 8:4-9. - PubMed
Gusev 2013
- Gusev EI, Martynov MYu, Kamtchatnov PR. Ischemic stroke: current status [Ishemichesky insult. Sovremennoye sostoyaniye problemy]. Nevrologiya [Neurology] 2013;83(5):2-7.
Guyatt 2008
Hao 2012
Higgins 2003
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org (accessed May 2023).
Hong 2009
- Hong Z, Moessler H, Bornstein N, Brainin M, Heiss W-D, CASTA Investigators. A double-blind, placebo-controlled, randomized trial to evaluate the safety and efficacy of Cerebrolysin in patients with acute ischaemic stroke in Asia - CASTA. International Journal of Stroke 2009;4(5):406-12. - PubMed
Horton 2019
ICH 2003
- ICH Expert Working Group. ICH Harmonised tripartite guideline. Post-approval safety data management: definitions and standards for expedited reporting E2D. database.ich.org/sites/default/files/E2D_Guideline.pdf 12 November 2003 (accessed May 2023).
Lachin 2016
Leonardi‐Bee 2007
Lindekleiv 2018
Liu 2016
Liu 2018
Makarenko 2005
- Makarenko AN, Kositsin NS, Nazimov IV, Svinov MM, Goloborod'ko EV, Pasikova NV. A comparative study of antistroke activity of the new drug "cerebral" and its fractions in rats [Сравнительное изучение антиинсультной активности церебрала и его отдельных субфракций в эксперименте]. Eksperimental'naia i Klinicheskaia Farmakologiia [Experimental and Clinical Pharmacology] 2005;68(2):15-20. - PubMed
Martynchik 2013
- Martynchik SA, Sokolova OV. Medical and economic assessment and rationale for improving organization of inpatient care for cerebral stroke [Mediko-ekonomicheskaya otsenka i obosnovaniye sovershenstvovaniya organizatsionnykh form okazaniya statsionarnoy pomoshchi pri mozgovom insul’te]. Sotsial’nyye aspekty zdorov’ya naseleniya [Social Aspects of Population Health ] 2013;30(2):e-pub without numbered pages. [vestnik.mednet.ru/content/view/473/30/lang,ru/]
Masliah 2012
MedDRA 2011
- MedDRA Maintenance and Support Services Organization. Introductory Guide to MedDRA Version 14.0. www.meddra.org/how-to-use/support-documentation (accessed prior to 23 June 2020).
Minhas 2022
MinHealthRu 2021
- Ministry of Healthcare of the Russian Federation. Cliniсal guidelines. Ischemic stroke and transient ischemic attack in adults [Клинические рекомендации. Ишемический инсульт и транзиторная ишемическая атака у взрослых]. https://cr.minzdrav.gov.ru/schema/171_2 2021 (accessed October 2022).
Molnar 2008
Muir 2003
Muresanu 2016
Onishchenko 2006
- Onishchenko LS, Gaikova ON, Ianishevskii SN. Changes in the focus of experimental ischemic stroke under the influence of neuroprotective drugs. Morfologiia [Morphology] 2006;130(6):40-6. - PubMed
Plavinski 2016
- Plavinsk SL, Shabalkin PI. A meta-analysis. Cerebrolisyn, Cortexin, Cellex, efficiency in vascular dementia, Alzheimer's disease and ischemic stroke [Мета-анализ. Церебролизин, Кортексин,Целлекс: эффективность при сосудистой деменции, болезни Альцгеймера и ишемическом инсульте]. In Rus. Zhurnal Medistina (Journal "Medicine") 2016;2:1-15. [https://fsmj.ru/01614.html]
Quinn 2021a
Quinn 2021b
Registry of Medicines 2019
- Registry of Medicines. Cerebrolysin [Церебролизин® (Cerebrolysin®)]. www.rlsnet.ru/tn_index_id_3528.htm (accessed prior to 23 June 2020).
Retraction Watch
- Marcus A, Oransky I. Retraction Watch. retractionwatch.com (accessed 8 June 2022).
Retraction Watch Database
- Marcus A, Oransky I. Retraction Watch Database. retractiondatabase.org/RetractionSearch.aspx? (accessed 8 June 2022).
Review Manager 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ricci 2012a
Ricci 2012b
Righetti 2004
Riley 2006
- Riley C, Hutter-Paier B, Windisch M, Doppler E, Moessler H, Wronski R. A peptide preparation protects cells in organotypic brain slices against cell death after glutamate intoxication. Journal of Neural Transmission 2006;113(1):103-10. - PubMed
Salim 2008
Sandercock 2009
Sandercock 2011
Sandercock 2014
Sandercock 2015
Sandercock 2017
Sapronov 2005
- Sapronov NS, Bul'on VV, Kuznetsova NN, Selina EN. The neuroprotector effect of a new taurine derivative on a model of compression spinal cord trauma in rats [Neyroprotektornyy effekt novogo proizvodnogo taurina pri kompressionnoy travme spinnogo mozga]. Eksperimental'naya i Klinicheskaya Farmakologiya [Experimental and Clinical Pharmacology] 2005;68(6):45-8. - PubMed
Schauer 2006
- Schauer E, Wronski R, Patockova J, Moessler H, Doppler E, Hutter-Paier B, et al. Neuroprotection of Cerebrolysin in tissue culture models of brain ischemia: post lesion application indicates a wide therapeutic window. Journal of Neural Transmission 2006;113(7):855-68. - PubMed
Schünemann 2011
- Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shamalov 2010
- Shamalov NA, Stakhovskaia LV, Burenchev DV, Kichuk IV, Tvorogova TV, Botsina AI, et al. The effect of Cerebrolysin in dosage 50 ml on the volume of lesion in ischemic stroke. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2010;110(12 Pt 2):34-7. [PMID: ] - PubMed
Shu 2012
- Shu LLS, San Jose CZ, Pasco PP. The efficacy and safety of cerebrolysin in acute hemorrhagic stroke: a meta analysis. Cerebrovascular Diseases 2012;34 Suppl 1:36.
Shuaib 2007
- Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, et al. NXY-059 for the treatment of acute ischemic stroke. New England Journal of Medicine 2007;357(6):562-71. - PubMed
Skvortsova 2006
- Skvortsova VI, Stakhovskaia LV, Shamolov NA, Kerbikov OB. Results of the multicenter prospective study of Cerebrolysin safety and efficacy in acute stroke. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2006;Suppl 16:41-5. [PMID: ] - PubMed
Suslina 2009
- Suslina ZA, Varakin YY, Vereshchagin NV. Clinical and epidemiological research - a promising area for the study of cerebrovascular pathology (first report) [Kliniko-epidemiologicheskiye issledovaniya – perspektivnoye napravleniye izucheniya tserebrovaskulyarnoy patologii (soobshcheniye pervoye)]. Annaly klinicheskoy i eksperimental’noy nevrologii [Annals of Clinical and Experimental Neurology] 2009;3(3):4-11.
TISC 2001
Vilenskiĭ 2006
- Vilenskii BS, Iakhno NN. The problem of cerebral stroke: its contemporary state. Vestnik Rossiiskoi Akademii Meditsinskikh Nauk [Bulletin of the Russian Academy of Medical Sciences] 2006;9-10:18-24. - PubMed
Visvanathan 2015
Wang 2017
Wang 2021
Wardlaw 2013
Wardlaw 2014
WHO 2019a
- World Health Organization. The Atlas of Heart Disease and Stroke. www.who.int/cardiovascular_diseases/resources/atlas/en/ (accessed November 2019).
WHO 2019b
- National Essential Medicines Lists. www.who.int/selection_medicines/country_lists (accessed November 2019).
WHO 2022
- WHO Global Essential Medicine Lists. https://global.essentialmeds.org/dashboard/medicines (accessed October 2022).
Wong 2005
Wu 2015
Yang 2015
Zeng 2005
Zhang 2010
Zhang 2019
Zhuo 2008
References to other published versions of this review
Ziganshina 2010a
Ziganshina 2010b
- Ziganshina LE, Abakumova T, Kuchaeva A. Cerebrolysin for acute ischaemic stroke. International Journal of Risk and Safety in Medicine 2010;22(4):179-93.
Ziganshina 2013
- Ziganshina LE, Abakumova TR. Cerebrolysin for acute ischemic stroke. Vestnik Rossiiskoi Akademii Meditsinskikh Nauk [Bulletin of the Russian Academy of Medical Sciences] 2013;1:21-9. - PubMed
Ziganshina 2015
Ziganshina 2016
Ziganshina 2017
Ziganshina 2020
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous