Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development - PubMed (original) (raw)
Review
Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development
Meenu Mehta et al. ACS Mater Au. 2023.
Abstract
Over the past decade, the therapeutic potential of nanomaterials as novel drug delivery systems complementing conventional pharmacology has been widely acknowledged. Among these nanomaterials, lipid-based nanoparticles (LNPs) have shown remarkable pharmacological performance and promising therapeutic outcomes, thus gaining substantial interest in preclinical and clinical research. In this review, we introduce the main types of LNPs used in drug formulations such as liposomes, nanoemulsions, solid lipid nanoparticles, nanostructured lipid carriers, and lipid polymer hybrid nanoparticles, focusing on their main physicochemical properties and therapeutic potential. We discuss computational studies and modeling techniques to enhance the understanding of how LNPs interact with therapeutic cargo and to predict the potential effectiveness of such interactions in therapeutic applications. We also analyze the benefits and drawbacks of various LNP production techniques such as nanoprecipitation, emulsification, evaporation, thin film hydration, microfluidic-based methods, and an impingement jet mixer. Additionally, we discuss the major challenges associated with industrial development, including stability and sterilization, storage, regulatory compliance, reproducibility, and quality control. Overcoming these challenges and facilitating regulatory compliance represent the key steps toward LNP's successful commercialization and translation into clinical settings.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Figure 1
Chemical structures of various components used in LNP formulations. These include phospholipids (1,2-dioleoyl-_sn_-glycero-3-phosphoethanolamine (DOPE), 1,2-dioleoyl-_sn_-glycero-3-phosphocholine (DOPC), and phosphatidylinositol), cholesterol, cationic and ionizable lipids (1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), (1,2-dilinoleyloxy-3-dimethylaminopropane) (Dlin-MC3-DMA)), and PEG lipids (1,2-distearoyl-_sn_-glycero-3-phosphoethanolamine-_N_-[methoxy(polyethylene glycol)](DSPE-PEG), 1,2-distearoyl-_sn_-glycero-3-phosphoethanolamine-_N_-[succinyl(polyethylene glycol)] (DSG-PEG)).
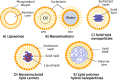
Figure 2
Schematic illustration depicts the structure of various LNP formulations used in drug delivery. (A) Liposomes are spherical vesicles with phospholipid bilayers, encapsulating hydrophilic drugs in their aqueous core and incorporating hydrophobic drugs within the lipid bilayers. (B) Nanoemulsions comprise oil droplets dispersed in an aqueous phase stabilized by surfactants, accommodating lipophilic drugs in the oil phase while preventing droplet aggregation. (C) Solid lipid nanoparticles (SLNs) consist of solid lipid matrices entrapping hydrophobic drugs, forming nanoscale particles with a lipid core. (D) Nanostructured lipid carriers (NLCs) are similar to SLNs but contain a combination of solid and liquid lipids, resulting in a more stable matrix and improved drug loading capacity. (E) Lipid polymer hybrid nanoparticles combine lipid-based and polymer-based components, offering the benefits of both systems. These nanoparticles can effectively encapsulate various types of drugs and exhibit enhanced stability and controlled release properties. Each type of LNP structure provides unique advantages and can be tailored for targeted drug delivery, enabling the encapsulation of a diverse range of therapeutic agents. Figure created with
BioRender.com
.
Figure 3
LNP synthesis processes in laboratory and industry environments: (A) Nanoprecipitation, (B) single/double emulsification, (C) nonsolvent emulsification, (D) thin film hydration, (E) microfluidic process, and (F) impingement jet mixer. Created with
BioRender.com
.
Figure 4
Different designs of microchips used in microfluidic devices: (A) Y-shaped mixer, (B) hydrodynamic flow focusing, (C) staggered herringbone mixer, and (D) bifurcating mixer. Created with
BioRender.com
.
Similar articles
- siRNA Functionalized Lipid Nanoparticles (LNPs) in Management of Diseases.
Kalita T, Dezfouli SA, Pandey LM, Uludag H. Kalita T, et al. Pharmaceutics. 2022 Nov 19;14(11):2520. doi: 10.3390/pharmaceutics14112520. Pharmaceutics. 2022. PMID: 36432711 Free PMC article. Review. - Chemistry of Lipid Nanoparticles for RNA Delivery.
Eygeris Y, Gupta M, Kim J, Sahay G. Eygeris Y, et al. Acc Chem Res. 2022 Jan 4;55(1):2-12. doi: 10.1021/acs.accounts.1c00544. Epub 2021 Dec 1. Acc Chem Res. 2022. PMID: 34850635 Review. - Lipid-Polymer Hybrid Nanosystems: A Rational Fusion for Advanced Therapeutic Delivery.
Jain S, Kumar M, Kumar P, Verma J, Rosenholm JM, Bansal KK, Vaidya A. Jain S, et al. J Funct Biomater. 2023 Aug 23;14(9):437. doi: 10.3390/jfb14090437. J Funct Biomater. 2023. PMID: 37754852 Free PMC article. Review. - Lipid nanoparticles (LNPs) for in vivo RNA delivery and their breakthrough technology for future applications.
Jeong M, Lee Y, Park J, Jung H, Lee H. Jeong M, et al. Adv Drug Deliv Rev. 2023 Sep;200:114990. doi: 10.1016/j.addr.2023.114990. Epub 2023 Jul 7. Adv Drug Deliv Rev. 2023. PMID: 37423563 Review. - Understanding the formation mechanism of lipid nanoparticles in microfluidic devices with chaotic micromixers.
Maeki M, Fujishima Y, Sato Y, Yasui T, Kaji N, Ishida A, Tani H, Baba Y, Harashima H, Tokeshi M. Maeki M, et al. PLoS One. 2017 Nov 28;12(11):e0187962. doi: 10.1371/journal.pone.0187962. eCollection 2017. PLoS One. 2017. PMID: 29182626 Free PMC article.
Cited by
- Natural Food Components as Biocompatible Carriers: A Novel Approach to Glioblastoma Drug Delivery.
Rajendran AT, Vadakkepushpakath AN. Rajendran AT, et al. Foods. 2024 Sep 4;13(17):2812. doi: 10.3390/foods13172812. Foods. 2024. PMID: 39272576 Free PMC article. Review. - Nanomaterial-Driven Precision Immunomodulation: A New Paradigm in Therapeutic Interventions.
Aljabali AAA, Obeid MA, Gammoh O, El-Tanani M, Mishra V, Mishra Y, Kapre S, Srivatsa Palakurthi S, Hassan SS, Nawn D, Lundstrom K, Hromić-Jahjefendić A, Serrano-Aroca Á, Redwan EM, Uversky VN, Tambuwala MM. Aljabali AAA, et al. Cancers (Basel). 2024 May 27;16(11):2030. doi: 10.3390/cancers16112030. Cancers (Basel). 2024. PMID: 38893150 Free PMC article. Review. - Recent advanced lipid-based nanomedicines for overcoming cancer resistance.
Dechbumroong P, Hu R, Keaswejjareansuk W, Namdee K, Liang XJ. Dechbumroong P, et al. Cancer Drug Resist. 2024 Jun 21;7:24. doi: 10.20517/cdr.2024.19. eCollection 2024. Cancer Drug Resist. 2024. PMID: 39050885 Free PMC article. Review. - Nanotechnology-driven therapies for neurodegenerative diseases: a comprehensive review.
López-Espinosa J, Park P, Holcomb M, Godin B, Villapol S. López-Espinosa J, et al. Ther Deliv. 2024;15(12):997-1024. doi: 10.1080/20415990.2024.2401307. Epub 2024 Sep 19. Ther Deliv. 2024. PMID: 39297726 Review. - Chemistry and Art of Developing Lipid Nanoparticles for Biologics Delivery: Focus on Development and Scale-Up.
John R, Monpara J, Swaminathan S, Kalhapure R. John R, et al. Pharmaceutics. 2024 Jan 19;16(1):131. doi: 10.3390/pharmaceutics16010131. Pharmaceutics. 2024. PMID: 38276502 Free PMC article. Review.
References
- Mittal D.; Kaur G.; Singh P.; Yadav K.; Ali S. A. Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Frontiers in Nanotechnology 2020, 2, Review.10.3389/fnano.2020.579954. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous