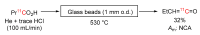Gas Phase Transformations in Carbon-11 Chemistry - PubMed (original) (raw)
Review
Gas Phase Transformations in Carbon-11 Chemistry
Shuiyu Lu et al. Int J Mol Sci. 2024.
Abstract
The short-lived positron-emitter carbon-11 (_t_1/2 = 20.4 min; β+, 99.8%) is prominent for labeling tracers for use in biomedical research with positron emission tomography (PET). Carbon-11 is produced for this purpose with a cyclotron, nowadays almost exclusively by the 14N(p,α)11C nuclear reaction, either on nitrogen containing a low concentration of oxygen (0.1-0.5%) or hydrogen (~5%) to produce [11C]carbon dioxide or [11C]methane, respectively. These primary radioactive products can be produced in high yields and with high molar activities. However, only [11C]carbon dioxide has some utility for directly labeling PET tracers. Primary products are required to be converted rapidly and efficiently into secondary labeling synthons to provide versatile radiochemistry for labeling diverse tracer chemotypes at molecular positions of choice. This review surveys known gas phase transformations of carbon-11 and summarizes the important roles that many of these transformations now play for producing a broad range of labeling synthons in carbon-11 chemistry.
Keywords: PET; carbon-11; catalysts; gas phase; on-line processes; radiochemistry; radiotracer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Scheme 1
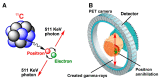
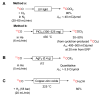
Decay of carbon-11 (A) and principle of PET (B). Reprinted and modified from Li and Conti, 2010 [3], with permission from Elsevier.
Scheme 2
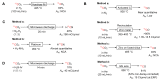
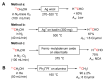
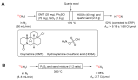
Processes leading to [11C]carbon dioxide and [11C]methane by proton irradiation of nitrogen gas with low level oxygen (A) or ~5% hydrogen (B), respectively. * Indicates hot atom or energetic species.
Scheme 3
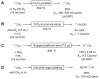
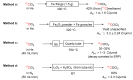
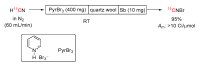
Conversions of [11C]carbon dioxide into other 11C-labeling agents: (A) [11C]methane; (B) [11C]carbon monoxide; (C) [11C]hydrogen cyanide; and (D) [11C]carbon disulfide.
Scheme 4
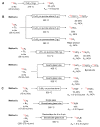
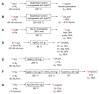
Conversion of [11C]methane into (A) [11C]carbon dioxide; (B) [11C]methanol; (C) [11C]hydrogen cyanide; and (D) [11C]acetylene.
Scheme 5
Halogenations of [11C]methane to produce useful labeling synthons: (A) fluorination; (B) chlorination; (C) bromination; and (D) iodination.
Scheme 5
Halogenations of [11C]methane to produce useful labeling synthons: (A) fluorination; (B) chlorination; (C) bromination; and (D) iodination.
Scheme 6
Conversions of [11C]carbon monoxide into other labeling synthons and intermediates: (A) [11C]phosgene; (B) [11C]carbonyl difluoride; and (C) proposed for [11C]methanol.
Scheme 7
Conversions of [11C]methanol into (A) [11C]formaldehyde and (B) [11C]iodomethane.
Scheme 8
Conversion of [1-11C]ethanol into [1-11C]ethylene.
Scheme 9
Conversions of [11C]carbon tetrachloride into [11C]phosgene.
Scheme 10
Metathetical conversions of [11C]haloalkanes into useful labeling synthons by passage over heated silver(I) or sodium salts into (A,B) [11C]methyl triflate; (C) [11C]iodomethane; (D) [11C]nitroalkanes; (E) [11C]methanethiol; (F) [11C]mesyl chloride; and (G) [_methyl_-11C]methyl isocyanate.
Scheme 11
Conversions of [11C]iodomethane into (A) [11C]hydrogen cyanide and (B) [11C]carbon disulfide.
Scheme 12
Conversion of [11C]hydrogen cyanide into [11C]cyanogen bromide.
Scheme 13
Conversion of [1-11C]butyric acid into [1-11C]propylketene.
Similar articles
- [11 C]Fluoroform, a Breakthrough for Versatile Labeling of PET Radiotracer Trifluoromethyl Groups in High Molar Activity.
Haskali MB, Pike VW. Haskali MB, et al. Chemistry. 2017 Jun 16;23(34):8156-8160. doi: 10.1002/chem.201701701. Epub 2017 May 17. Chemistry. 2017. PMID: 28514059 Free PMC article. - Recent Developments in Carbon-11 Chemistry and Applications for First-In-Human PET Studies.
Pees A, Chassé M, Lindberg A, Vasdev N. Pees A, et al. Molecules. 2023 Jan 17;28(3):931. doi: 10.3390/molecules28030931. Molecules. 2023. PMID: 36770596 Free PMC article. Review. - Radiolabeling with [11C]HCN for Positron emission tomography.
Zhou YP, Makaravage KJ, Brugarolas P. Zhou YP, et al. Nucl Med Biol. 2021 Nov-Dec;102-103:56-86. doi: 10.1016/j.nucmedbio.2021.09.002. Epub 2021 Sep 25. Nucl Med Biol. 2021. PMID: 34624831 Free PMC article. Review. - Carbon-11 and fluorine-18 chemistry devoted to molecular probes for imaging the brain with positron emission tomography.
Dollé F. Dollé F. J Labelled Comp Radiopharm. 2013 Mar-Apr;56(3-4):65-7. doi: 10.1002/jlcr.3037. J Labelled Comp Radiopharm. 2013. PMID: 24285311 - 11CO2 fixation: a renaissance in PET radiochemistry.
Rotstein BH, Liang SH, Holland JP, Collier TL, Hooker JM, Wilson AA, Vasdev N. Rotstein BH, et al. Chem Commun (Camb). 2013 Jun 25;49(50):5621-9. doi: 10.1039/c3cc42236d. Epub 2013 May 14. Chem Commun (Camb). 2013. PMID: 23673726 Free PMC article. Review.
References
- Ferrieri R.A., Wolf A.P. The chemistry of positron emitting nucleogenic (hot) atoms with regard to preparation of labelled compounds of practical utility. Radiochim. Acta. 1983;34:69–84. doi: 10.1524/ract.1983.34.12.69. - DOI
- Ferrieri R.A. Handbook of Radiopharmaceuticals—Radiochemistry and Applications. John Wiley & Sons; Hoboken, NJ, USA: 2002. Production and Application of Synthetic Precursors Labeled with Carbon-11 and Fluorine-18; pp. 229–282. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources