Effect of Hydroxychloroquine and Azithromycin Combination Use in COVID-19 Patients - An Umbrella Review - PubMed (original) (raw)
Review
Effect of Hydroxychloroquine and Azithromycin Combination Use in COVID-19 Patients - An Umbrella Review
Kaushik Nag et al. Indian J Community Med. 2024 Jan-Feb.
Abstract
Background: Hydroxychloroquine and Azithromycin combination was used rampantly in management of COVID-19 patients in different countries. Present review was conducted to evaluate the efficacy of Hydroxychloroquine and Azithromycin combination compared to the control (standard care) and any adverse effect following this combination use in COVID-19 patients if any.
Material and methods: We included all the systematic review with or without meta-analysis reporting the effect of Hydroxychloroquine (HCQ) and Azithromycin (AZM) combination use in COVID-19 patient using three databases namely PubMed, medline, CINHAL, Web of Science from July 2020 till Jan 2022.
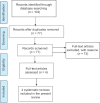
Results: The systematic search strategy has identified 104 studies in total, after removal of duplicates only 4 systematic reviews were included in the qualitative synthesis. The various tools for assessing and reporting the data in the reviews were PRISMA, ROBINS-I, Robs2, AMSTAR, MASTER checklists. Mortality among the hydroxychloroquine with azithromycin combination group was significantly higher than among the Standard Care group. The duration of hospital stay in days was shorter in the Standard Care group in comparison with the hydroxychloroquine group or the hydroxychloroquine and azithromycin combination group. Of the 4 systematic reviews included, 3 had low risk of bias and one had unclear risk of bias using the ROBIS tool. Chloroquine or Hydroxychloroquine combination did not shorten the duration of hospital stay.
Conclusion: Rampant use of Chloroquine or Hydroxychloroquine alone or with Azithromycin combination caused adverse effects like QT prolongation. Finally, there is no evidence to support use of either Hydroxychloroquine with or without Azithromycin, for the treatment of COVID-19.
Keywords: Azithromycin; COVID-19; PRISMA; hydroxychloroquine; patients; umbrella review.
Copyright: © 2024 Indian Journal of Community Medicine.
Conflict of interest statement
There are no conflicts of interest.
Figures
Figure 1
PRISMA flow chart
Similar articles
- Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19.
Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T. Singh B, et al. Cochrane Database Syst Rev. 2021 Feb 12;2(2):CD013587. doi: 10.1002/14651858.CD013587.pub2. Cochrane Database Syst Rev. 2021. PMID: 33624299 Free PMC article. - A systematic review and meta-analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID-19 treatment.
Ghazy RM, Almaghraby A, Shaaban R, Kamal A, Beshir H, Moursi A, Ramadan A, Taha SHN. Ghazy RM, et al. Sci Rep. 2020 Dec 17;10(1):22139. doi: 10.1038/s41598-020-77748-x. Sci Rep. 2020. PMID: 33335141 Free PMC article. - Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis.
Fiolet T, Guihur A, Rebeaud ME, Mulot M, Peiffer-Smadja N, Mahamat-Saleh Y. Fiolet T, et al. Clin Microbiol Infect. 2021 Jan;27(1):19-27. doi: 10.1016/j.cmi.2020.08.022. Epub 2020 Aug 26. Clin Microbiol Infect. 2021. PMID: 32860962 Free PMC article. - Systematic review and meta-analysis of efficacy and safety of hydroxychloroquine and chloroquine in the treatment of COVID-19.
Mittal N, Mittal R, Gupta MC, Kaushal J, Chugh A, Khera D, Singh S. Mittal N, et al. J Family Med Prim Care. 2021 Jun;10(6):2126-2139. doi: 10.4103/jfmpc.jfmpc_2338_20. Epub 2021 Jul 2. J Family Med Prim Care. 2021. PMID: 34322403 Free PMC article. Review. - Chloroquine and hydroxychloroquine for COVID-19: Perspectives on their failure in repurposing.
Shah RR. Shah RR. J Clin Pharm Ther. 2021 Feb;46(1):17-27. doi: 10.1111/jcpt.13267. Epub 2020 Sep 27. J Clin Pharm Ther. 2021. PMID: 32981089 Free PMC article. Review.
References
- Chang T-H, Wang Li-F, Lin Y-S, Yang C-H, Yu C-Y, Lin Y-L. Hydroxychloroquine activates host antiviral innate immunity. Cytokine. 2014;70:33–4.
Publication types
LinkOut - more resources
Full Text Sources