Bone-Modifying Agents in Patients With High-Risk Metastatic Castration-Sensitive Prostate Cancer Treated With Abiraterone Acetate - PubMed (original) (raw)
Clinical Trial
Bone-Modifying Agents in Patients With High-Risk Metastatic Castration-Sensitive Prostate Cancer Treated With Abiraterone Acetate
Wataru Fukuokaya et al. JAMA Netw Open. 2024.
Abstract
Importance: The association between the use of bone-modifying agents (BMAs) and the outcomes among patients with metastatic castration-sensitive prostate cancer (mCSPC) treated with abiraterone acetate plus prednisone (AAP) remains unclear.
Objective: To investigate the association between BMA use and the outcomes of patients with mCSPC receiving AAP.
Design, setting, and participants: In this cohort study, a post hoc analysis of individual participant data from the LATITUDE trial was performed. The LATITUDE trial, a phase 3 randomized clinical trial, aimed to assess the efficacy of AAP and androgen deprivation therapy (ADT) vs dual-placebo and ADT in patients with high-risk mCSPC (data cutoff, August 15, 2018). Eligible patients had newly diagnosed prostate cancer with metastases and at least 2 of 3 high-risk factors (Gleason score ≥8, presence of ≥3 lesions on bone scan, or presence of measurable visceral metastasis). The trial was conducted at 235 sites in 34 countries. Data for the present study were evaluated from July 18 to September 23, 2023.
Exposures: Use of BMAs was defined as the administration of bisphosphonates and denosumab within 90 days before and after randomization.
Main outcomes and measures: The primary outcomes were time to skeletal-related events (SREs) and overall survival (OS). An SRE was defined as a clinical or pathological fracture, spinal cord compression, palliative radiation to bone, or surgery involving bone. Differences in these outcomes were examined using the restricted mean survival time from inverse probability of treatment weighting-adjusted Kaplan-Meier curves, estimated until the last event was observed (longest time observed, 63.9 months). Treatment × covariate interactions were analyzed using weighted Cox proportional hazards regression models for the total cohort.
Results: In the total cohort of 1199 patients (956 [79.7%] younger than 75 years), 597 (49.8%) received AAP and ADT, including 474 (79.4%) younger than 75 years and 384 (64.3%) with more than 10 bone metastases (AAP cohort); 602 (50.2%) were treated with dual placebo and ADT, including 482 (80.1%) younger than 75 years and 377 (62.6%) with more than 10 bone metastases (ADT cohort). In the AAP cohort, 132 patients (22.1%) received BMAs, while in the ADT cohort, 131 (21.8%) did. Zoledronic acid was the most frequently administered BMA in both the AAP (93 [70.5%]) and the ADT (88 [67.2%]) cohorts. During the median follow-up of 51.8 (IQR, 47.2-57.0) months in the AAP cohort, BMA use was associated with a longer time to SRE (difference, 7.8 [95% CI, 4.2-11.3] months) but not with OS (difference, 1.6 [95% CI, -2.5 to 5.8] months). In the ADT cohort, BMA use was associated with both time to SRE (difference, 9.3 [95% CI, 5.2-13.3] months) and OS (difference, 5.5 [95% CI, 3.2-9.8] months). No evidence was found that the outcomes of BMA varied by AAP or ADT (hazard ratio for time to SRE, 0.99 [95% CI, 0.48-2.08]; P = .99 for interaction; hazard ratio for OS, 1.31 [95% CI, 0.88-1.96]; P = .18 for interaction).
Conclusions and relevance: The findings of this cohort study suggest that use of BMAs was associated with a longer time to SRE in patients with high-risk mCSPC treated with ADT, with or without AAP, suggesting that BMA use might provide benefits to this population.
Conflict of interest statement
Conflict of Interest Disclosures: Dr Fukuokaya reported receiving grants-in-aid for scientific research from Japan Society for the Promotion of Science outside the submitted work. Dr Igarashi reported nonfinancial support from Jikei University Hospital during the conduct of the study and personal fees from the University of Pittsburgh outside the submitted work. Dr Kimura reported consulting for Astellas Pharma, Bayer AG, Janssen Pharmaceuticals, and Sanofi SA. No other disclosures were reported.
Figures
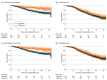
Figure 1.. Crude and Inverse Probability of Treatment Weighting (IPTW)–Adjusted Kaplan-Meier Curves Based on Bone-Modifying Agent (BMA) Use in the Abiraterone Acetate Plus Prednisone Cohort
Shaded areas indicate 95% CIs. OS indicates overall survival; SRE, skeletal-related event.
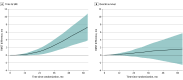
Figure 2.. Differences in Restricted Mean Survival Times Between Bone-Modifying Agent Users and Nonusers in the Abiraterone Acetate Plus Prednisone Cohort
Differences were calculated as restricted mean survival time for body-modifying agent users – restricted mean survival time for nonusers. A difference greater than 0 indicates longer survival in users, whereas a difference less than 0 indicates longer survival in nonusers. Shaded areas indicate 95% CIs.
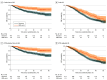
Figure 3.. Crude and Inverse Probability of Treatment Weighting (IPTW)–Adjusted Kaplan-Meier Curves Based on Bone-Modifying Agent (BMA) Use in the Androgen Deprivation Therapy Cohort
Shaded areas indicate 95% CIs. OS indicates overall survival; SRE, skeletal-related event.
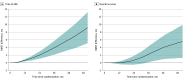
Figure 4.. Differences in Restricted Mean Survival Times Between Bone-Modifying Agent Users and Nonusers in the Androgen Deprivation Therapy Cohort
Differences were calculated as restricted mean survival time for body-modifying agent users – restricted mean survival time for nonusers. A difference greater than 0 indicates longer survival in users, whereas a difference less than 0 indicates longer survival in nonusers. Shaded areas indicate 95% CIs.
Similar articles
- Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, Sulur G, Luna Y, Li S, Mundle S, Chi KN. Fizazi K, et al. Lancet Oncol. 2019 May;20(5):686-700. doi: 10.1016/S1470-2045(19)30082-8. Epub 2019 Apr 12. Lancet Oncol. 2019. PMID: 30987939 Clinical Trial. - Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): an international, randomised phase 3 trial.
Chi KN, Protheroe A, Rodríguez-Antolín A, Facchini G, Suttman H, Matsubara N, Ye Z, Keam B, Damião R, Li T, McQuarrie K, Jia B, De Porre P, Martin J, Todd MB, Fizazi K. Chi KN, et al. Lancet Oncol. 2018 Feb;19(2):194-206. doi: 10.1016/S1470-2045(17)30911-7. Epub 2018 Jan 8. Lancet Oncol. 2018. PMID: 29326030 Clinical Trial. - Correlation of Prostate-specific Antigen Kinetics with Overall Survival and Radiological Progression-free Survival in Metastatic Castration-sensitive Prostate Cancer Treated with Abiraterone Acetate plus Prednisone or Placebos Added to Androgen Deprivation Therapy: Post Hoc Analysis of Phase 3 LATITUDE Study.
Matsubara N, Chi KN, Özgüroğlu M, Rodriguez-Antolin A, Feyerabend S, Fein L, Alekseev BY, Sulur G, Protheroe A, Li S, Mundle S, De Porre P, Tran N, Fizazi K. Matsubara N, et al. Eur Urol. 2020 Apr;77(4):494-500. doi: 10.1016/j.eururo.2019.11.021. Epub 2019 Dec 13. Eur Urol. 2020. PMID: 31843335 Clinical Trial. - Adding abiraterone to androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: A systematic review and meta-analysis.
Rydzewska LHM, Burdett S, Vale CL, Clarke NW, Fizazi K, Kheoh T, Mason MD, Miladinovic B, James ND, Parmar MKB, Spears MR, Sweeney CJ, Sydes MR, Tran N, Tierney JF; STOPCaP Abiraterone Collaborators. Rydzewska LHM, et al. Eur J Cancer. 2017 Oct;84:88-101. doi: 10.1016/j.ejca.2017.07.003. Epub 2017 Aug 8. Eur J Cancer. 2017. PMID: 28800492 Free PMC article. Review. - Hormone naïve metastatic prostate cancer: How to treat it?
Østergren PB, Ternov KK, Jensen CFS, Jakobsen H, Lindberg H, Sønksen J, Fode M. Østergren PB, et al. Arch Esp Urol. 2019 Mar;72(2):192-202. Arch Esp Urol. 2019. PMID: 30855021 Review. English.
References
- Surveillance, Epidemiology, and End Results Program . Cancer stat facts: prostate cancer. Accessed September 24, 2023. https://seer.cancer.gov/statfacts/html/prost.html
- Baek YH, Jeon HL, Oh IS, Yang H, Park J, Shin JY. Incidence of skeletal-related events in patients with breast or prostate cancer-induced bone metastasis or multiple myeloma: a 12-year longitudinal nationwide healthcare database study. Cancer Epidemiol. 2019;61:104-110. doi:10.1016/j.canep.2019.05.013 - DOI - PubMed
- European Association of Urology . Prostate cancer. 2023. Accessed September 24, 2023. https://uroweb.org/guidelines/prostate-cancer
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous