The recombinant shingles vaccine is associated with lower risk of dementia - PubMed (original) (raw)
The recombinant shingles vaccine is associated with lower risk of dementia
Maxime Taquet et al. Nat Med. 2024 Oct.
Abstract
There is emerging evidence that the live herpes zoster (shingles) vaccine might protect against dementia. However, the existing data are limited and refer only to the live vaccine, which is now discontinued in the United States and many other countries in favor of a recombinant vaccine. Whether the recombinant shingles vaccine protects against dementia remains unknown. Here we used a natural experiment opportunity created by the rapid transition from the use of live to the use of recombinant vaccines to compare the risk of dementia between vaccine types. We show that the recombinant vaccine is associated with a significantly lower risk of dementia in the 6 years post-vaccination. Specifically, receiving the recombinant vaccine is associated with a 17% increase in diagnosis-free time, translating into 164 additional days lived without a diagnosis of dementia in those subsequently affected. The recombinant shingles vaccine was also associated with lower risks of dementia than were two other vaccines commonly used in older people: influenza and tetanus-diphtheria-pertussis vaccines. The effect was robust across multiple secondary analyses, and was present in both men and women but was greater in women. These findings should stimulate studies investigating the mechanisms underpinning the protection and could facilitate the design of a large-scale randomized control trial to confirm the possible additional benefit of the recombinant shingles vaccine.
© 2024. The Author(s).
Conflict of interest statement
J.A.T. is a consultant for GSK and co-director of the Oxford-GSK Institute for Molecular and Computational Medicine. GSK had no involvement of any kind in this study and were not aware of it until after the paper was accepted. The other authors declare no competing interests.
Figures
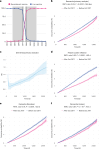
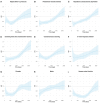
Fig. 1. Association between recombinant shingles vaccine and risk of dementia within 6 years of vaccination.
a, Proportion (in %) of each vaccine being received, showing the step change that occurred in October 2017. The exposure windows used in the primary analysis are shown in gray, and the restricted exposure windows that were used in a robustness analysis are shown in dark gray. b, Curves representing the Kaplan–Meier estimates of the cumulative incidence of dementia diagnosis in the 3 months to 6 years after shingles vaccination in the primary analysis (n = 103,837 in each cohort). c, Curve representing the time-varying hazard ratio (HR) for the risk of dementia in the primary analysis (HR < 1 indicates a lower risk of dementia in those who received the vaccine after October 2017); n = 103,837 in each cohort. d, Curves representing the Kaplan–Meier estimates of the cumulative incidence for herpes zoster infection (n = 103,837 in each cohort). e,f, Curves representing the Kaplan–Meier estimates of the cumulative incidence of dementia among women (e) and men (f) (n = 54,846 in each cohort for females, and n = 43,990 for males). The RMTL ratio, the P value (obtained using the _z_-test defined in the SurvRM2 package in R, two-sided and not corrected for multiple comparisons) for the association and the additional time that affected people lived diagnosis-free are reported above each figure. The exact P values are 2.9 × 10−15 (b), 4.3 × 10−41 (d) and 2.3 × 10−15 (e). Shaded areas in b–f represent 95% CIs of the cumulative incidences (b,d–f) or the time-varying HR (c).
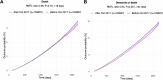
Extended Data Fig. 1. Kaplan-Meier curves for secondary outcomes in the 3 months-6 years after shingles vaccination in the primary analysis.
The curves represent the Kaplan-Meier estimates of the cumulative incidence of death (a) and the composite outcome of death or dementia (b). Shaded areas around curves represent 95% confidence intervals. n = 103,837 in each cohort. P-values were obtained using the z-test defined in the SurvRM2 package in R, two-sided and not corrected for multiple comparisons. The exact p-values for (B) is 3.8×10−7.
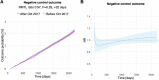
Extended Data Fig. 2. Results for the negative control outcome.
(a) Curves representing the Kaplan-Meier estimates of the cumulative incidence of the negative control outcome in the 3 months-6 years after shingles vaccination. n = 103,837 in each cohort. The p-value was obtained using the z-test defined in the SurvRM2 package in R, two-sided and not corrected for multiple comparisons. (b) Curve representing the time-varying hazard ratio for the negative control outcome (n = 103,837 in each cohort). Shaded areas in A and B represent 95% confidence intervals.
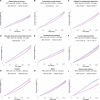
Extended Data Fig. 3. Kaplan-Meier curves showing the cumulative incidence of outcomes in the 3 months-6 years after shingles vaccination.
(a-h) Results for dementia in the different robustness analyses. In (A), the results correspond to the coarsened exact matching with pairwise alignment of follow-up horizons. (i) Results for the incidence of herpes zoster infection. The ratio of restricted mean time lost (RMTL), the p-value for the association, and the additional time lived diagnosis-free among affected people are reported above each figure. Curves in all panels represent the Kaplan-Meier estimates of the cumulative incidence of the corresponding outcome. Shaded areas in all panels represent 95% confidence intervals. See Supplementary Tables 5–9 for baseline characteristics. The number of individuals in each cohort was respectively (A) 82102, (B) 100532, (C) 110062, (D) 66998, (E) 82102, (F) 20243, (G) 54846, (H) 43990, (I) 103837. P-values were obtained using the z-test defined in the SurvRM2 package in R (except for (A) where it was obtained using bootstrap with 1000 repetitions and is reported as <0.001 because all bootstrap replicates of the ratio of RMTL were below 1), two-sided and not corrected for multiple comparisons. The exact p-values are (B) 7.5×10−16, (C) 1.4×10−14, (D) 1.5×10−17, (E) 1.6×10−11, (G) 2.3×10−15, and (I) 4.3×10−41.
Extended Data Fig. 4. Kaplan-Meier curves for the comparisons between shingles vaccines and two other vaccines: influenza and Tdap.
(a-b) Comparison with the recombinant shingles vaccine. (c-d) Comparison with live shingles vaccine. The ratio of restricted mean time lost (RMTL), the number of patients in each cohort, the bootstrap p-value for the association, and the additional time lived diagnosis-free among people affected are reported above each figure. Curves in all panels represent the Kaplan-Meier estimates of the cumulative incidence of the corresponding outcome. Shaded areas in all panels represent 95% confidence intervals. Baseline characteristics for these comparisons are provided in Supplementary Tables 11–14. The number of individuals in each cohort was respectively (A) 209031, (B) 98353, (C) 41466, and (D) 64035. P-values were obtained using the z-test defined in the SurvRM2 package in R, two-sided and not corrected for multiple comparisons. The exact p-values are (A) 1.4×10−67, (B) 2.6×10−53, (C) 1.2×10−6, and (D) 2.1×10−9.
Extended Data Fig. 5. Time-varying hazard ratios (HR).
Each curve represents the value of the HR from 3 months to 6 years post-vaccination. In (A), the results correspond to the coarsened exact matching with pairwise alignment of follow-up horizons. A HR < 1 indicates the risk is lower in those vaccinated predominantly with the recombinant vaccine. The shaded areas around the curves represent 95% CI. The number of individuals in each cohort was respectively (A) 82102, (B) 100532, (C) 110062, (D) 66998, (E) 82102, (F) 20243, (G) 54846, (H) 43990, (I) 103837.
Similar articles
- Vaccines for preventing herpes zoster in older adults.
Gagliardi AM, Andriolo BN, Torloni MR, Soares BG, de Oliveira Gomes J, Andriolo RB, Canteiro Cruz E. Gagliardi AM, et al. Cochrane Database Syst Rev. 2019 Nov 7;2019(11):CD008858. doi: 10.1002/14651858.CD008858.pub4. Cochrane Database Syst Rev. 2019. PMID: 31696946 Free PMC article. Updated. - Public health impact of herpes zoster vaccination on older adults in Hong Kong.
Chan PKS, Wong MCS, Chan M, Ching K, Giannelos N, Ng C. Chan PKS, et al. Hum Vaccin Immunother. 2023 Dec 31;19(1):2176065. doi: 10.1080/21645515.2023.2176065. Epub 2023 Feb 28. Hum Vaccin Immunother. 2023. PMID: 36854447 Free PMC article. - The adjuvanted recombinant zoster vaccine co-administered with a tetanus, diphtheria and pertussis vaccine in adults aged ≥50 years: A randomized trial.
Strezova A, Lal H, Enweonye I, Campora L, Beukelaers P, Segall N, Heineman TC, Schuind AE, Oostvogels L. Strezova A, et al. Vaccine. 2019 Sep 16;37(39):5877-5885. doi: 10.1016/j.vaccine.2019.08.001. Epub 2019 Aug 20. Vaccine. 2019. PMID: 31443993 Clinical Trial. - Vaccines for preventing herpes zoster in older adults.
de Oliveira Gomes J, Gagliardi AM, Andriolo BN, Torloni MR, Andriolo RB, Puga MEDS, Canteiro Cruz E. de Oliveira Gomes J, et al. Cochrane Database Syst Rev. 2023 Oct 2;10(10):CD008858. doi: 10.1002/14651858.CD008858.pub5. Cochrane Database Syst Rev. 2023. PMID: 37781954 Free PMC article. Review. - A practitioner's guide to the recombinant zoster vaccine: review of national vaccination recommendations.
Parikh R, Widenmaier R, Lecrenier N. Parikh R, et al. Expert Rev Vaccines. 2021 Sep;20(9):1065-1075. doi: 10.1080/14760584.2021.1956906. Epub 2021 Sep 1. Expert Rev Vaccines. 2021. PMID: 34311643 Review.
Cited by
- MAD-microbial (origin of) Alzheimer's disease hypothesis: from infection and the antimicrobial response to disruption of key copper-based systems.
Min JH, Sarlus H, Harris RA. Min JH, et al. Front Neurosci. 2024 Oct 2;18:1467333. doi: 10.3389/fnins.2024.1467333. eCollection 2024. Front Neurosci. 2024. PMID: 39416952 Free PMC article. Review. - The effect of herpes zoster vaccination at different stages of the disease course of dementia: Two quasi-randomized studies.
Xie M, Eyting M, Bommer C, Ahmed H, Geldsetzer P. Xie M, et al. medRxiv [Preprint]. 2024 Aug 23:2024.08.23.24312457. doi: 10.1101/2024.08.23.24312457. medRxiv. 2024. PMID: 39228711 Free PMC article. Preprint.
References
- Eyting, M., Xie, M., Heß, S. & Geldsetzer, P. Causal evidence that herpes zoster vaccination prevents a proportion of dementia cases. Preprint at medRxiv10.1101/2023.05.23.23290253 (2023).
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- NIHR203316/DH | National Institute for Health Research (NIHR)
- 4-SRA-2017-473-A-N/JDRF/United States
- 203141/A/16/Z/Wellcome Trust (Wellcome)
LinkOut - more resources
Full Text Sources
Medical