The ribotoxic stress response drives acute inflammation, cell death, and epidermal thickening in UV-irradiated skin in vivo - PubMed (original) (raw)
. 2024 Dec 19;84(24):4774-4789.e9.
doi: 10.1016/j.molcel.2024.10.044. Epub 2024 Nov 25.
Zhenzhen Wu 2, Muhammad Jasrie Firdaus 3, Goda Snieckute 2, Gee Ann Toh 3, Malin Jessen 4, José Francisco Martínez 2, Peter Haahr 5, Thomas Levin Andersen 6, Melanie Blasius 2, Li Fang Koh 7, Nina Loeth Maartensson 8, John E A Common 9, Mads Gyrd-Hansen 4, Franklin L Zhong 10, Simon Bekker-Jensen 11
Affiliations
- PMID: 39591967
- PMCID: PMC11671030
- DOI: 10.1016/j.molcel.2024.10.044
The ribotoxic stress response drives acute inflammation, cell death, and epidermal thickening in UV-irradiated skin in vivo
Anna Constance Vind et al. Mol Cell. 2024.
Abstract
Solar UVB light causes damage to the outermost layer of skin. This insult induces rapid local responses, such as dermal inflammation, keratinocyte cell death, and epidermal thickening, all of which have traditionally been associated with DNA damage response signaling. Another stress response that is activated by UVB light is the ribotoxic stress response (RSR), which depends on the ribosome-associated mitogen-activated protein 3 kinases (MAP3K) ZAKα and culminates in p38 and JNK activation. Using ZAK knockout mice, we here show that it is the RSR that is responsible for the early manifestation of UVB-induced skin inflammation and keratinocyte death and subsequent proliferation in vivo. We also show that the RSR controls both p38-mediated pyroptotic and JNK-mediated apoptotic programmed cell death of human keratinocytes in vitro. In sum, our work highlights that skin cells rely on a cytoplasmic and ribosomal stress signal rather than a nuclear and DNA-templated signal for rapid inflammatory responses to UV exposure.
Keywords: JNK; UV; ZAK-alpha; apoptosis; inflammation; p38; pyroptosis; ribotoxic stress response; skin.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
Graphical abstract
Figure 1
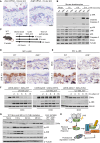
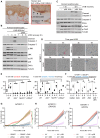
The ribotoxic stress response is activated in mouse skin by UVB irradiation (A) Paraffin-embedded mouse skin was stained for Zakα (left) and Zakβ (right) mRNA (pink dots) by in situ hybridization with a modified RNAScope protocol. The dashed line demarcates the epidermis from the dermis. (B) Keratinocytes isolated from tail skin of WT and ZAK−/− mice were UVB irradiated (500 J/m2) and left to recover for the indicated times. Lysates were analyzed by immunoblotting with the indicated antibodies. (C) Schematic of in vivo mouse UVB-irradiation experiments. 9-week-old female WT and ZAK−/− mice were shaved on their backs and their skin was depilated 48 h prior to UVB exposure (500 J/m2). Different times of euthanasia and skin harvesting are indicated. (D) Skins of mice from (C) were harvested 4 h after irradiation and analyzed by immunohistochemistry for activated p38 (p-p38). (E) As in (D), except that p-JNK antibody was used. (F) U2OS cells deleted for ZAK (ΔZAK) were stably rescued with doxycycline (Dox)-inducible WT and mutated forms of ZAKα. Cells were UVB irradiated (500 J/m2) and left to recover for the indicated times. Lysates were analyzed as in (B). (G) Full-thickness skin of WT mice irradiated as in (C) was lysed and analyzed as in (B). Liver lysates from WT and ZAK−/− mice served as a control for antibody specificity. (H) Model of activation-associated ZAKα degradation by the SCFβ-Trcp ubiquitin ligase and the proteasome. All scale bars, 50 μm. See also Figure S1.
Figure 2
ZAK−/− mice are protected against early immune cell infiltration in UVB-irradiated skin (A) Schematic of in vivo mouse UVB-irradiation experiment for fluorescence-activated cell sorting (FACS)-based analysis of skin-infiltrating immune cells. 9-week-old female WT and ZAK−/− mice were shaved on their backs and their skin was depilated 48 h prior to UVB exposure (500 J/m2). Mice were euthanized and back skin was harvested 6 h after irradiation. (B–D) Mouse skin samples were collected from control or UVB-irradiated mice and immune infiltrates were analyzed by flow cytometry. Graphs represent the mean number of (B) total immune cells (CD45+), (C) monocytes (CD11b+ Ly-6C+), and (D) neutrophils (CD11b+ Ly-6G+). The data were obtained from two independent experiments with 3–7 mice per group. Data are presented as mean, with error bars denoting the standard error of the mean (SEM). ns., non-significant; ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p < 0.001; ∗∗∗∗p ≤ 0.0001 using two-way ANOVA with the Sidak method. (E) Skin sections from (A) were analyzed by immunohistochemistry for myeloperoxidase (MPO) expression. Red arrows indicate MPO-positive immune cells. (F) Representative images of female mice 24 and 48 h after UVB irradiation (500 J/m2). (G) Severity of skin inflammation of 9-week-old female WT and ZAK−/− mice at 6, 8, 24, and 48 h after UVB irradiation (500 J/m2) was assessed histologically based on infiltrating immune cells according to the following scoring system: normal, 0; intraluminal infiltration, 1; intraluminal + light dermal infiltration, 2; intraluminal + moderate dermal infiltration, 3. Data are plotted as mean and all error bars represent the standard error of the mean (SEM) (n = 3–9 biological replicates). ns., non-significant; ∗p ≤ 0.05; ∗∗p ≤ 0.01; in two-way ANOVA with the Sidak method. (H) Hematoxylin and eosin (H&E)-stained skin sections from (A). Red arrows indicate intraluminal infiltration and yellow asterisks indicate dermal infiltration of immune cells. All scale bars, 50 μm. See also Figures S1–S3.
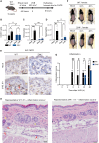
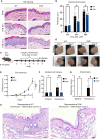
Figure 3
The ribotoxic stress response drives epidermal thickening in mouse skin (A) Representative H&E-stained images of UVB-irradiated skin (500 J/m2) harvested from 9-week-old female WT and ZAK−/− mice after 24 and 48 h of recovery. Notice the attenuated thickening of the epithelial cell layer in ZAK−/− mice. (B) Quantification of (A)—average thickness of the epidermal cell layer. (C) Schematic of in vivo mouse anisomycin exposure experiment. Anisomycin was dissolved in ethanol (vehicle—2 mg/mL) and applied to one ear of 8- to 10-week-old female WT and ZAK−/− mice for 5 consecutive days. Pure ethanol was similarly applied to the other ear and mice were euthanized on day 6. (D) Appearance of mouse ears subjected to the protocol in (C) before euthanasia. Notice the inflamed and irritated appearance of the WT ear after 5 days of anisomycin application. (E) Growth in ear thickness of mice from (C) was measured with a caliper. Only data from anisomycin-treated ears are shown. Data are plotted as mean and all error bars represent the standard error of the mean (SEM). ∗∗∗∗p ≤ 0.0001; in one-way ANOVA at the 6-day time point. (F) Epidermal cell layer thickness of ears from (C) were measured as in (B). (G) Severity of skin inflammation in ears from (B) was assessed histologically based on infiltrating immune cells according to the scoring system described in the legend of Figure 2G. (H) Representative H&E-stained images of ears from (C). All data are plotted as mean and all error bars represent the standard error of the mean (SEM) (n = 3–10 biological replicates). ns., non-significant; ∗p ≤ 0.05; in two-way ANOVA with the Sidak method. All scale bars, 50 μm. See also Figure S4.
Figure 4
UVB-induced apoptosis and pyroptosis is ZAK dependent in human keratinocytes (A) Paraffin-embedded human skin was analyzed for ZAKα expression by immunohistochemistry. Scale bar, 50 μm. (B) Primary human keratinocytes were UVB irradiated (500 J/m2) in the presence of ZAK inhibitor (ZAKi, 1 μM) and harvested at indicated time points. Lysates were analyzed by immunoblotting with the indicated antibodies. (C) as in (B), except that cells were treated with inhibitors (i, 2 μM) of p38 and JNK kinases. (D) Representative bright-field microscopy images of morphology to distinguish pyroptosis (pink arrows) and apoptosis (blue arrows) in N/TERT-1 cells following UVB exposure (100 J/m2) at the indicated time points. Scale bars, 10 μm. (E) Quantification of the different types of cell death seen in (D). ns., non-significant; ∗∗∗∗p ≤ 0.0001; in one-way ANOVA. (F) Quantification of apoptotic ΔNLRP1 N/TERT-1 cells treated with indicated inhibitors (i, 0.5 μM) and UVB irradiation (100 J/m2). ns., non-significant; ∗p ≤ 0.05; ∗∗p ≤ 0.01; in one-way ANOVA. (G–I) N/TERT-1 cells were grown in the presence of the live-cell-staining dyes DRAQ7 (permeabilized cells) and pSIVA (apoptosis marker). Cells were treated with indicated inhibitors (i, 1 μM), irradiated with UVB (100 J/m2), and subjected to fluorescence time-lapse microscopy. Error bars are from 3 technical replicates that are representative of 2 independent repeats. Statistical significance was calculated by two-tailed Kolmogorov-Smirnov test. Pyroptosis by DRAQ7 curve between 0 and 5 h, and apoptosis by pSIVA curve between 5 and 14 h. ns., nonsignificant; ∗∗∗∗p ≤ 0.0001. See also Figure S5.
Figure 5
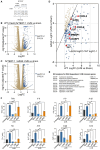
RNA sequencing reveals ZAK-dependent and UVB-induced transcriptional signatures in human keratinocytes (A) Design of RNA sequencing (RNA-seq) experiment using indicated genotypes of N/TERT-1 cells. (B) Significantly upregulated transcripts by UVB in WT/Cas9 control cells shown in blue. (C) Significantly upregulated transcripts by UVB in ZAK KO/Cas9 control cells shown in blue. (D) Correlation plot of UVB-induced transcripts in WT vs. ZAK KO cells. We selected transcripts that underwent a smaller log2-transformed fold change (logFC) in ZAK KO cells than in WT cells, as indicated by the diagonal at y = x − 1. Well-known chemokines and cytokines and cell-death-related genes are highlighted. (E) Gene Ontology (GO) analysis of ZAK-dependent and UVB-induced transcripts. (F) Primary human keratinocytes from one donor were UVB irradiated (500 J/m2, 6 h) in the presence of inhibitors (i, 2 μM) against the kinases ZAK, p38, and JNK, as indicated. qPCR analysis of isolated RNA was performed using primers against the indicated transcripts. Fold changes are plotted as mean and all error bars represent the standard deviation (SD) (n = 3 technical replicates). ns., non-significant; ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p < 0.001; ∗∗∗∗p ≤ 0.0001; in two-way ANOVA with the Sidak method. See also Figure S6.
Figure 6
The DNA damage response does not impact on early UVB-induced keratinocyte cell death (A) N/TERT-1 cells were treated with ZAK and ATR inhibitors (i, 1 μM), UVB irradiated (100 J/m2), and lysed at the indicated time points. Lysates were analyzed by immunoblotting with the indicated antibodies. (B) Primary human keratinocytes from one donor were UVB irradiated (500 J/m2, 6 h) in the presence of ZAK and ATR inhibitors (i, 2 μM), as indicated. qPCR analysis of isolated RNA was performed using primers against the indicated transcripts. Fold changes are plotted as mean and all error bars represent the standard deviation (SD) (n = 3 technical replicates). ∗∗∗p < 0.001; ∗∗∗∗p ≤ 0.0001; in two-way ANOVA with the Sidak method. (C) N/TERT-1 cells were treated as in (A) and lysed after 8 h. Lysates were analyzed by immunoblotting with the indicated antibodies. (D) N/TERT-1 cells were grown in the presence of the live-cell-staining dyes DRAQ7 (permeabilized cells) and pSIVA (apoptosis marker). Cells were treated with ATR inhibitor (i, 1 μM), irradiated with UVB (100 J/m2), and subjected to fluorescence time-lapse microscopy. Statistical significance was calculated by two-tailed Kolmogorov-Smirnov test at 95% confidence interval. Pyroptosis by DRAQ7 curve between 0 and 5 h, and apoptosis by pSIVA curve between 5 and 14 h. ns., nonsignificant. (E) N/TERT-1 cells deleted for DDB2 (ΔDDB2) were treated with ZAK inhibitor (i, 1 μM) and UVB irradiated (100 J/m2—8 h recovery). Lysates were analyzed as in (A). (F) As in (E), except that cells were deleted for ERCC2 (ΔERCC2). (G–I) Cells from (E) and (F) were grown in the presence of the live-cell-staining dye DRAQ7, treated with indicated inhibitors (i, 1 μM), irradiated with UVB (100 J/m2), and subjected to fluorescence time-lapse microscopy. Statistical significance for pyroptosis was calculated by two-tailed Kolmogorov-Smirnov test between 0 and 5 h. ns., non-significant; ∗∗∗p < 0.001; ∗∗∗∗p ≤ 0.0001. (J) Stable rescue of U2OS/ΔZAK cells with WT and mutated forms of ZAKα (protein domains and mutations are annotated in Figure S7F). Cells were irradiated with UVB (500 J/m2—1 h) and lysates were analyzed as in (A). (K) WT, ΔZAK, and ΔSLFN11 HAP1 cells were irradiated with UVB (500 J/m2) and analyzed as in (G)–(I). Data are plotted as mean and all error bars represent the standard error of the mean (SEM). ∗∗p ≤ 0.01; ∗∗∗p < 0.001; in two-way ANOVA at the 14-h time point. (D, G–I, and K) Error bars are from 3 technical replicates that are representative of 1–3 independent repeats. See also Figure S7.
Figure 7
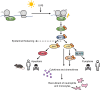
The ribotoxic stress response orchestrates early skin responses to UVB irradiation In skin keratinocytes, UVB-induced mRNA damage causes ribosome stalling and ensuing collision, providing an activation platform for ZAKα. Downstream, ribotoxic stress response (RSR) signaling mediates rapid p38-dependent pyroptotic cell death in human keratinocytes (right arm) and slower, JNK-dependent apoptotic cell death in mouse and human keratinocytes (left arm). RSR signaling also mediates p38 and MK2-dependent secretion of inflammatory cytokines and chemokines. These events underlie UVB-induced skin infiltration of immune cells and thickening of the epidermal cell layer.
Similar articles
- Based on network pharmacology, molecular docking and experimental verification to reveal the mechanism of Andrographis paniculata against solar dermatitis.
Deng Q, Chen W, Deng B, Chen W, Chen L, Fan G, Wu J, Gao Y, Chen X. Deng Q, et al. Phytomedicine. 2024 Dec;135:156025. doi: 10.1016/j.phymed.2024.156025. Epub 2024 Sep 7. Phytomedicine. 2024. PMID: 39326136 - Cyanidin-3-glucoside inhibits UVB-induced oxidative damage and inflammation by regulating MAP kinase and NF-κB signaling pathways in SKH-1 hairless mice skin.
Pratheeshkumar P, Son YO, Wang X, Divya SP, Joseph B, Hitron JA, Wang L, Kim D, Yin Y, Roy RV, Lu J, Zhang Z, Wang Y, Shi X. Pratheeshkumar P, et al. Toxicol Appl Pharmacol. 2014 Oct 1;280(1):127-37. doi: 10.1016/j.taap.2014.06.028. Epub 2014 Jul 22. Toxicol Appl Pharmacol. 2014. PMID: 25062774 Free PMC article. - ZBP1-mediated PANoptosis is a crucial lethal form in diverse keratinocyte death modalities in UVB-induced skin injury.
Bi X, Li M, Guo Y, Hu M, Chen Y, Lian N, Chen S, Li M, Gu H, Chen X. Bi X, et al. Cell Death Dis. 2025 Jan 26;16(1):44. doi: 10.1038/s41419-025-07351-3. Cell Death Dis. 2025. PMID: 39863598 Free PMC article. - Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.
Bryant A, Hiu S, Kunonga PT, Gajjar K, Craig D, Vale L, Winter-Roach BA, Elattar A, Naik R. Bryant A, et al. Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review. - Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.
Triana L, Palacios Huatuco RM, Campilgio G, Liscano E. Triana L, et al. Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials