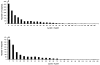Levels of lipoprotein(a), apolipoprotein B, and lipoprotein cholesterol distribution in IDDM. Results from follow-up in the Diabetes Control and Complications Trial - PubMed (original) (raw)
Clinical Trial
Levels of lipoprotein(a), apolipoprotein B, and lipoprotein cholesterol distribution in IDDM. Results from follow-up in the Diabetes Control and Complications Trial
J Q Purnell et al. Diabetes. 1995 Oct.
Abstract
Levels of lipoprotein(a) [Lp(a)], apolipoprotein (apo) B, and lipoprotein cholesterol distribution using density-gradient ultracentrifugation were measured as part of a cross-sectional study at the final follow-up examination (mean 6.2 years) in the Diabetes Control and Complications Trial. Compared with the subjects in the conventionally treated group (n = 680), those subjects receiving intensive diabetes therapy (n = 667) had a lower level of Lp(a) (Caucasian subjects only, median 10.7 vs 12.5 mg/dl, respectively; P = 0.03), lower apo B (mean 83 vs. 86 mg/dl, respectively; P = 0.01), and a more favorable distribution of cholesterol in the lipoprotein fractions as measured by density-gradient ultracentrifugation with less cholesterol in the very-low-density lipoprotein and the dense low-density lipoprotein fractions and greater cholesterol content of the more buoyant low-density lipoprotein. Compared with a nondiabetic Caucasian control group (n = 2,158), Lp(a) levels were not different in the intensive treatment group (median 9.6 vs. 10.7 mg/dl, respectively; NS) and higher in the conventional treatment group (9.6 vs. 12.5 mg/dl, respectively; P < 0.01). No effect of renal dysfunction as measured by increasing albuminuria or reduced creatinine clearance on Lp(a) levels could be demonstrated in the diabetic subjects. Prospective follow-up of these subjects will determine whether these favorable lipoprotein differences in the intensive treatment group persist and whether they influence the onset of atherosclerosis in insulin-dependent diabetes.
Figures
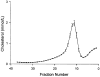
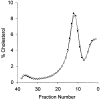
Figure 1
Mean distribution of lipoprotein cholesterol across nonequilibrium DGUC (line) and 1 SD (bars) of 15 repeat runs on a single sample stored at −70 degrees C for 12–24 months
Figure 2
Frequency distribution histogram of Lp(a) levels in 1,299 Caucasian IDDM patients (A) and 2,158 Caucasian control subjects (B).
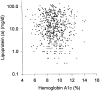
Figure 3
Relationship between Lp(a) level and HbA1c in the Caucasian subjects of the conventionally treated group (n = 653). r = 0.052; P = 0.18.
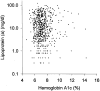
Figure 4
Relationship between Lp(a) level and HbA1c in the Caucasian subjects of the intensively treated group (n = 646). r = 0.022; P = 0.59.
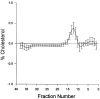
Figure 5
Distribution of lipoprotein cholesterol across nonequilibrium density-gradient ultracentrifugation of the conventionally treated group [open bullet] (n = 698) and the intensively treated group [bullet] (n = 680). HDL is located in fractions 0–6, LDL in fractions 7 to approximately 18, IDL in fractions 19–30, and VLDL in fractions 31–38.
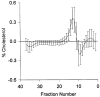
Figure 6
Difference plot comparing the mean cholesterol levels of each fraction from the DGUC for differences from 0, with 95 percent CI (bars), between the intensively and conventionally treated groups. The fractional location of each of the lipoprotein classes (VLDL, IDL, LDL, and HDL) is the same as in Figure 3.
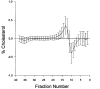
Figure 7
Difference plot comparing the mean cholesterol levels of each fraction for differences from 0, with 95 percent CI (bars), for intensively treated women (n = 323) versus conventionally treated women (n = 315). The location of each of the lipoprotein classes (VLDL, IDL, LDL, and HDL) is the same as in Figure 3.
Figure 8
Difference plot comparing the mean cholesterol levels of each fraction for differences from 0, with 95 percent CI (bars), for intensively treated men (n = 357) versus conventionally treated men (n = 383). The location of each of the lipoprotein classes (VLDL, IDL, LDL, and HDL) is the same as in Figure 3.
Similar articles
- Serum lipids and lipoprotein(a) concentrations in Chinese NIDDM patients. Relation to metabolic control.
Chang CJ, Kao JT, Wu TJ, Lu FH, Tai TY. Chang CJ, et al. Diabetes Care. 1995 Aug;18(8):1191-4. doi: 10.2337/diacare.18.8.1191. Diabetes Care. 1995. PMID: 7587858 - Response of apolipoprotein AIV and lipoproteins to glycaemic control in young people with insulin-dependent diabetes mellitus.
Attia N, Touzani A, Lahrichi M, Balafrej A, Kabbaj O, Girard-Globa A. Attia N, et al. Diabet Med. 1997 Mar;14(3):242-7. doi: 10.1002/(SICI)1096-9136(199703)14:3<242::AID-DIA340>3.0.CO;2-1. Diabet Med. 1997. PMID: 9088774 - Lipid and lipoprotein patterns in type 2 non-obese diabetic patients. Do Lp(a) levels decrease with improved glycemic control in these patients?
Alagözlü H, Gültekin F, Candan F. Alagözlü H, et al. Nutr Metab Cardiovasc Dis. 2000 Aug;10(4):204-8. Nutr Metab Cardiovasc Dis. 2000. PMID: 11079258 - Intraperitoneal insulin infusion improves the depletion in choline-containing phospholipids of lipoprotein B particles in type I diabetic patients.
Guerci B, Igau B, Ziegler O, Crea T, Fruchart JC, Drouin P, Fievet C. Guerci B, et al. Metabolism. 1996 Apr;45(4):430-4. doi: 10.1016/s0026-0495(96)90215-2. Metabolism. 1996. PMID: 8609827 Clinical Trial.
Cited by
- The Roles of Fatty Acids and Apolipoproteins in the Kidneys.
Pan X. Pan X. Metabolites. 2022 May 20;12(5):462. doi: 10.3390/metabo12050462. Metabolites. 2022. PMID: 35629966 Free PMC article. Review. - Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study.
Diabetes Prevention Program Outcomes Study Research Group; Orchard TJ, Temprosa M, Barrett-Connor E, Fowler SE, Goldberg RB, Mather KJ, Marcovina SM, Montez M, Ratner RE, Saudek CD, Sherif H, Watson KE. Diabetes Prevention Program Outcomes Study Research Group, et al. Diabet Med. 2013 Jan;30(1):46-55. doi: 10.1111/j.1464-5491.2012.03750.x. Diabet Med. 2013. PMID: 22812594 Free PMC article. Clinical Trial. - Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial.
Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Purnell JQ, et al. JAMA. 1998 Jul 8;280(2):140-6. doi: 10.1001/jama.280.2.140. JAMA. 1998. PMID: 9669786 Free PMC article. Clinical Trial. - Cardiovascular Risk in Type 1 Diabetes Mellitus.
Schofield J, Ho J, Soran H. Schofield J, et al. Diabetes Ther. 2019 Jun;10(3):773-789. doi: 10.1007/s13300-019-0612-8. Epub 2019 Apr 19. Diabetes Ther. 2019. PMID: 31004282 Free PMC article. Review. - Lipoprotein (a): impact by ethnicity and environmental and medical conditions.
Enkhmaa B, Anuurad E, Berglund L. Enkhmaa B, et al. J Lipid Res. 2016 Jul;57(7):1111-25. doi: 10.1194/jlr.R051904. Epub 2015 Dec 4. J Lipid Res. 2016. PMID: 26637279 Free PMC article. Review.
References
- Krolewski AS, Kosinski EJ, Warram JH, Leland OS, Busick EJ, Asmal AC, Rand LI, Christlieb AR, Bradley RF, Kahn CR. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59:750–755. - PubMed
- Jensen T, Borch-Johnsen K, Kofoed-Enevoldsen A, Deckard T. Coronary heart disease in young type I (insulin dependent) diabetic patients with and without diabetic nephropathy: incidence and risk factors. Diabetologia. 1987;30:144–148. - PubMed
- Consensus Statement of the American Diabetes Association. Role of cardiovascular risk factors in prevention and treatment of macrovascular disease in diabetes. Diabetes Care. 1989;12:537–579. Library Holdings. - PubMed
- The Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol. 1995;75:894–903. - PubMed
- Austin MA, Hokanson JE. Epidemiology of triglycerides, small dense low-density lipoprotein, and lipoprotein (a) as risk factors for coronary heart disease. Med Clin North Am. 1994;78:99–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous