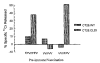Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen - PubMed (original) (raw)
. 1995 May 1;154(9):4685-92.
Affiliations
- PMID: 7722321
- PMCID: PMC1976248
Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen
M Wang et al. J Immunol. 1995.
Abstract
Some tumor cells express Ags that are potentially recognizable by T lymphocytes and yet do not elicit significant immune responses. To explore new immunotherapeutic strategies aimed at enhancing the recognition of these tumor-associated Ags (TAA), we developed an experimental mouse model consisting of a lethal clone of the BALB/c tumor line CT26 designated CT26.WT, which was transduced with the lacZ gene encoding beta-galactosidase, to create CT26.CL25. The growth rate and lethality of CT26.CL25 and CT26.WT were virtually identical despite the expression by CT26.CL25 of the model tumor Ag in vivo. A recombinant fowlpox virus (rFPV), which is replication incompetent in mammalian cells, was constructed that expressed the model TAA, beta-galactosidase, under the influence of the 40-kDa vaccinia virus early/late promoter. This recombinant, FPV.bg40k, functioned effectively in vivo as an immunogen, eliciting CD8+ T cells that could effectively lyse CT26.CL25 in vitro. FPV.bg40k protected mice from both subcutaneous and intravenous tumor challenge by CT26.CL25, and most surprisingly, mice bearing established 3-day pulmonary metastasis were found to have significant, Ag-specific decreases in tumor burden and prolonged survival after treatment with the rFPV. These observations constitute the first reported use of rFPV in the prevention and treatment of an experimental cancer and suggest that changing the context in which the immune system encounters a TAA can significantly and therapeutically alter the host immune response against cancer.
Figures
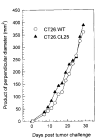
Fig. 1
Unirradiated BALB/c mice (five per group) were injected with 3 × l05 tumor cells, both CT26.WT and CT26.CL25, in the right flank, and the product of the largest perpendicular diameters was calculated from measurements beginning on day 7 and every other day thereafter for a total of 12 measurements. The measurements were made in a coded, blinded fashion, and the values represent the average of five mice. The average deviation of the measurements has SD less than 10%. The experiment was repeated with similar results.
Fig. 2. BALB/c mice were injected with either 5 × 105 or 1 × l07 PFU of FPV.wt or rFPV.bg40k
Twenty-one days later, splenocytes from all the immunized mice and unimmunized mice (None) were restimulated with 1 _μ_g/ml of synthetic peptide TPHPARIGL, for 7 days and then assayed for specific lysis in a 51Cr release assay against CT26.WT, CT26.WT pulsed with TPHPARICL, CT26.CL25, or EL4.E22 (an H-2b tumor that expresses _β_-gal). Spontaneous release for all reported 51Cr release assays is less than 10%. The experiment was repeated with similar results.
Fig. 3
Day 0, BALB/c mice immunized with 5 × 105 or 1 × l07 PFU of rFPV.40k or FPV.C. Day 21, the same mice were either challenged with CT26.W or CT26.CL25. Mice that developed tumors larger than 10 mrn in a single diameter were considered to have developed lethal tumors. All surviving mice have no palpable tumors. The figure represents two experiments each with six mice per group. The experiment was repeated with similar results.
Fig. 4. BALB/c mice were injected i.v. with either 5 × 105 CT26.WT or CT26.CL25
On day 3 after tumor challenge, mice were immunized i.v. with either 5 × 105 or 1 × 107 PFU of FPV.wt or rFPV.bg40k, or s.c. with either 5 × 105 irradiated (10,000 rad) CT26.CL25 or 5 × 105 CT26.CL25 admixed with 50 _μ_g of C. parvum, or 0.5 ml of HBSS. On day 12 after tumor challenge, lungs were harvested, and the number of pulmonary metastases were enumerated in a coded, blinded fashion. Each data point represents an individual animal. Open circles represent animals bearing CT26.WT tumors, whereas closed circles represent animals bearing CT26.CL25. The experiment was repeated with similar results.
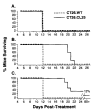
Fig. 5. BALB/c mice were challenged i.v. with 5 × 105 CT26.WT or CT26.CL25
Mice were treated with HBSS (A), with one immunization with 1 × 107 PFU of FPV.bg40k on day 3 (B), or with two immunizations with 1 × 107 PFU of rFPV.bg40k on days 3 and day 10 (C). All mice treated with either one or twod oses of FPV.wt were found dead by day 12 after treatment (not shown). Mice were checked every day after day 12 for survival. The experiment was repeated with similar results.
Fig. 6
BALB/c mice were immunized with 1 × 107 FPV.wt or 5 × 106 VV.wt and 14 days after the first immunization, a second immunization with either 1 × 107 PFU of FPV.bg40k or 5 × 106 PFU of HPV-E6Vac (_β_-gal-expressing VV). On day 28, splenocytes from all micew ere restimulated in culture with 1 _μ_M synthetic peptide TPHPARIGL for 7 days and then assayed for specific lysis in a 51Cr release assay against CT26.WT or CT26.CL25. Results for an effector-to-target ratio of 25:1 are shown. The experiment was repeated with similar results.
Similar articles
- Anti-tumor activity of cytotoxic T lymphocytes elicited with recombinant and synthetic forms of a model tumor-associated antigen.
Wang M, Chen PW, Bronte V, Rosenberg SA, Restifo NP. Wang M, et al. J Immunother Emphasis Tumor Immunol. 1995 Oct;18(3):139-46. doi: 10.1097/00002371-199510000-00001. J Immunother Emphasis Tumor Immunol. 1995. PMID: 8770769 Free PMC article. - Highly attenuated modified vaccinia virus Ankara (MVA) as an effective recombinant vector: a murine tumor model.
Carroll MW, Overwijk WW, Chamberlain RS, Rosenberg SA, Moss B, Restifo NP. Carroll MW, et al. Vaccine. 1997 Mar;15(4):387-94. doi: 10.1016/s0264-410x(96)00195-8. Vaccine. 1997. PMID: 9141209 Free PMC article. - IL-2 enhances the function of recombinant poxvirus-based vaccines in the treatment of established pulmonary metastases.
Bronte V, Tsung K, Rao JB, Chen PW, Wang M, Rosenberg SA, Restifo NP. Bronte V, et al. J Immunol. 1995 May 15;154(10):5282-92. J Immunol. 1995. PMID: 7730632 Free PMC article. - Prospects and limitations of recombinant poxviruses for prostate cancer immunotherapy.
Hwang C, Sanda MG. Hwang C, et al. Curr Opin Mol Ther. 1999 Aug;1(4):471-9. Curr Opin Mol Ther. 1999. PMID: 11713762 Review. - Recombinant fowlpox virus vaccines for poultry.
Boyle DB, Heine HG. Boyle DB, et al. Immunol Cell Biol. 1993 Oct;71 ( Pt 5)(5):391-7. doi: 10.1038/icb.1993.45. Immunol Cell Biol. 1993. PMID: 8270268 Free PMC article. Review.
Cited by
- Cellular and vascular effects of the photodynamic agent temocene are modulated by the delivery vehicle.
García-Díaz M, Kawakubo M, Mroz P, Sagristà ML, Mora M, Nonell S, Hamblin MR. García-Díaz M, et al. J Control Release. 2012 Sep 10;162(2):355-63. doi: 10.1016/j.jconrel.2012.07.025. Epub 2012 Jul 27. J Control Release. 2012. PMID: 22841794 Free PMC article. - Evaluation of prime/boost regimens using recombinant poxvirus/tyrosinase vaccines for the treatment of patients with metastatic melanoma.
Lindsey KR, Gritz L, Sherry R, Abati A, Fetsch PA, Goldfeder LC, Gonzales MI, Zinnack KA, Rogers-Freezer L, Haworth L, Mavroukakis SA, White DE, Steinberg SM, Restifo NP, Panicali DL, Rosenberg SA, Topalian SL. Lindsey KR, et al. Clin Cancer Res. 2006 Apr 15;12(8):2526-37. doi: 10.1158/1078-0432.CCR-05-2061. Clin Cancer Res. 2006. PMID: 16638862 Free PMC article. Clinical Trial. - Raman Spectroscopy and Machine Learning Reveals Early Tumor Microenvironmental Changes Induced by Immunotherapy.
Paidi SK, Rodriguez Troncoso J, Raj P, Monterroso Diaz P, Ivers JD, Lee DE, Avaritt NL, Gies AJ, Quick CM, Byrum SD, Tackett AJ, Rajaram N, Barman I. Paidi SK, et al. Cancer Res. 2021 Nov 15;81(22):5745-5755. doi: 10.1158/0008-5472.CAN-21-1438. Epub 2021 Oct 13. Cancer Res. 2021. PMID: 34645610 Free PMC article. - The new vaccines: building viruses that elicit antitumor immunity.
Restifo NP. Restifo NP. Curr Opin Immunol. 1996 Oct;8(5):658-63. doi: 10.1016/s0952-7915(96)80082-3. Curr Opin Immunol. 1996. PMID: 8902391 Free PMC article. Review. - Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors.
Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Duraiswamy J, et al. Cancer Res. 2013 Jun 15;73(12):3591-603. doi: 10.1158/0008-5472.CAN-12-4100. Epub 2013 Apr 30. Cancer Res. 2013. PMID: 23633484 Free PMC article.
References
- Boon T, Cerottini J, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337. - PubMed
- Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL., Jr Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716. - PubMed
- Pardoll DM. Tumour antigens. A new look for the 1990s. Nuture. 1994;369:357. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials